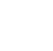A-1 Introduction
Cyanobacteria, also known as blue-green algae, are a group of microorganisms that live in freshwater and marine habitats throughout the world. Several cyanobacteria species have the ability to produce cyanotoxins, which pose a threat to human health, especially for those who directly consume water and fish taken from a water body with a high concentration of cyanobacteria. Please see Section 3 in HCB-1 and Section 2.2 in HCB-2 for important information about health concerns associated with exposure to cyanobacteria blooms.
Technically, cyanobacteria are bacteria; however, the names algal bloom and harmful algal bloom (HAB) have persisted for many decades. In this document, we specifically refer to blooms composed of cyanobacteria as harmful cyanobacterial blooms (HCBs).
Planktonic HCBs typically form under specific conditions, such as abundant nutrients, a stable water column, ample light, and warm temperatures. Most planktonic cyanobacteria also regulate their buoyancy to optimize their position in the water column or float to the surface. Wind can disrupt this process and allow massive accumulations of organisms on the leeward shoreline of a water body. See HCB-1 Section 3.3 for more information.
Benthic cyanobacteria may occur in any water body but are frequently found in those with clear water and abundant light at depth. They can obtain nutrients from the sediment and therefore may occur in low-nutrient waters. They are typically attached but may also float and accumulate downstream/downwind. See Section 1.3 in HCB-2 for more information on ecology of benthic HCBs.
Warming global temperatures may increase cyanobacteria bloom prevalence and impacts. One reason is because these blooms proliferate at very warm water temperatures and are more tolerant of these warmer conditions than their competitors, such as green algae. In addition, warming temperatures and less ice cover are creating a longer growing period, or the length of time when a water body is above the temperature threshold that favors cyanobacteria.
A-2 Using This Guide
Water bodies contain many organisms or natural phenomena that can be mistaken for HCBs. This guide is intended to help you evaluate potential HCBs in the field using their visual appearance and basic microscopy. This guide illustrates the typical appearance of both planktonic and benthic cyanobacteria and non-cyanobacteria accumulations. This guide can be used in conjunction with the Field and Laboratory Guide to Freshwater Cyanobacteria Harmful Algal Blooms for Native American and Alaska Native Communities (Rosen and St. Amand 2015). The following references were used in creating this guide: Wacklin, Hoffmann, and Komárek (2009), Komárek (2013), Komárek et al. (2014), Komárek and Anagnostidis (2001, 2005), Otsuka et al. (2001), and Wehr, Sheath, and Kociolet (2014).
Cyanobacteria taxonomy and our understanding of cyanotoxins and harmful compounds produced by individual cyanobacteria taxa are constantly evolving. Information presented here is current as of the date of publication. Health information presented here should be compared to sources that are frequently updated with new data, such as the Centers for Disease Control and Prevention (CDC 2021) and U.S. Environmental Protection Agency (USEPA 2021) cyanobacteria web pages.
Updated taxonomic names usually take time to be adopted, with both old and new names used simultaneously in outreach materials and by the press. Here we present common taxa with the currently accepted name first, followed by the historical name in parentheses—for example, Dolichospermum (Anabaena). For more information on taxonomic usage in this guide and for a crosswalk table of new versus old names, please refer to Section A-9.
This Visual Guide to Common Harmful Cyanobacteria is organized into several sections:
- Planktonic HCBs that form monospecific blooms (Section A-5): Microscopic images by genus/species and images showing their visual appearance in the field, along with a description of their microscopic features, associated cyanotoxins, and growth habits
- Planktonic cyanobacteria that form mixed-assemblage blooms (Section A-6): Microscopic images by genus/species of taxa that seldom form extensive monospecific blooms yet may be found in your sample
- Benthic cyanobacteria, both attached and floating (Section A-7): Microscopic images by genus/species and images showing their visual appearance in the field
- Nontoxic surface and subsurface accumulations (Section A-8): Microscopic images and corresponding field images of organisms commonly mistaken for HCBs
Skill level needed to successfully use this guidance. This guide is intended for use by lake managers and others who may not be trained HCB taxonomists. Non-experts can use the visual appearances of phenomena observed in water bodies to separate potential HCBs from blooms formed by common organisms other than cyanobacteria. These organisms are not individually visible to the naked eye, and a microscope is required to correctly determine what type of bloom is present. Non-experts with basic microscopy skills can use this document to further distinguish between HCBs and other common aquatic organisms; however, samples or photographs should also be provided to a taxonomist for confirmation whenever possible. For those learning to identify cyanobacteria in the field, we have also developed a video—Learning to Identify Cyanobacteria Blooms —available on the ITRC YouTube channel.
Note: This guide was developed as a web-based document. Some features may not be available if it is accessed offline or via a PDF copy.
A-3 Health and Safety
HCBs can produce cyanotoxins and other compounds that are harmful to people and animals. Accidental ingestion or skin contact should be avoided with any material suspected to include cyanobacteria. Aerosols and odors produced by cyanobacteria may also be harmful. You should take precautions to avoid exposure:
- Protect your skin: Wear gloves, clothing, and footwear that do not bring you in contact with suspect material or water.
- Avoid accidental ingestion: Wash your hands and gear that may have been in contact with suspect material or water. Do not store your samples in areas where food is also stored.
- Keep children and pets away from suspect material and water.
- Sensitivity to cyanobacteria may be different for each individual and can change over time. Monitor your health and contact your doctor if you believe you may be ill after exposure to potential HCBs.
A-4 Glossary
- Aerotope: Clusters of gas vesicles that provide buoyancy and give the cell a brownish appearance when numerous.
- Akinete: An enveloped, thick-walled, nonmotile, dormant cell formed by filamentous cyanobacteria under the order Nostocales and Stigonematales. Akinetes are resistant to cold and dehydration, and they can be viable for decades.
- Attenuated: End cells in the trichomes that become narrower or taper.
- Bright field: Unenhanced microscopic image. Unless otherwise noted, microscopic images in this guide are bright field images.
- Filament: Trichome including a sheath.
- Heterocyte (heterocyst): A differentiated cyanobacterial cell that carries out nitrogen fixation. Heterocytes provide an anaerobic environment, then use a 60-enzyme complex to fix atmospheric nitrogen, converting it to ammonia that is transported to adjacent cells.
- Heteropolar: Trichomes that are morphologically different from one end to the other.
- Intercalary: Mid-filament location.
- Isodiametric: Cells are about as long as wide within the trichome.
- Isopolar: Trichomes are morphologically the same from one end to the other.
- Keritomy: Net-like appearance of protoplast caused by arrangement of thylakoids or net-like nucleoids, or by the tendency to form intra-thylakoidal spaces.
- Nomarski: Optical microscopy technique used to enhance contrast in transparent samples.
- Paraheterocytically: Located in the middle of the filament.
- Phase: Optical microscopy technique that enhances contrast in transparent or colorless samples.
- Pro-akinete: Immature akinete cell.
- Sheath: Cyanobacterial sheaths consist of a meshwork of polysaccharide fibrils that surround both individual cells and entire colonies. Sheaths may appear concentric or layered in certain genera. They can be colored (especially with age) and involved in motility.
- Terminal: Located at the end of a filament.
- Thylakoid: Thylakoid membranes contain cyanobacterial pigments and are the site of both photosynthesis and respiration. They are typically layered near the periphery cyanobacteria cells.
- Trichome: Uniseriate or multiseriate arrangements of cells that form a single longitudinal or branched structural unit. Cells can be shorter or longer than wide, as well as straight, twisted, or flexed
A-5 Planktonic HCBs That Form Monospecific Blooms: Field Images and Microscopic Images
These field photos illustrate the color, texture, and general appearance of planktonic HCBs. We include examples from diverse locations around the country whenever possible. This section focuses on planktonic taxa for which we have both field photos and confirmed identification of the main HCB taxa present.
HCB appearance varies with the taxa and with age. Planktonic HCBs and material washing onto the shoreline are exposed to sunlight, heat, and waves, which can damage cells and release their contents. In addition to the green, green-blue, red, and brown coloration of healthy cyanobacteria, damaged cells may appear turquoise and white as chlorophyll breaks down. Foams may also develop when cell contents are whipped up by waves. Note that foam is also formed when aquatic plants or other algae die.

Figure A-1. Foam accumulated along the shoreline, Lake Champlain, VT.
Source: Angela Shambaugh. Used with permission.

Figure A-2. Foam associated with an aquatic plant die-off, Lake Champlain, VT.
Source: Angela Shambaugh. Used with permission.
The following pages contain photos of blooms and microscopic cyanobacteria images. Photos are sorted by genus and presented alphabetically. Where possible, several photos are included to give you a better idea of the range of possibilities for each taxon. It is also important to keep in mind that while the photos presented in this guide are focused on the taxa of concern, these taxa often occur alongside or intermingled with other aquatic phenomena.
Aphanizomenon flos-aquae – Bloom images

Figure A-3. Aphanizomenon flos-aquae, Klamath Lake, OR.
Source: Jacob Kann.

Figure A-4. Aphanizomenon flos-aquae, Klamath Lake, OR.
Source: Jacob Kann.

Figure A-5. Aphanizomenon flos-aquae, with green clouds on the right, WI.
Source: Ann St. Amand.
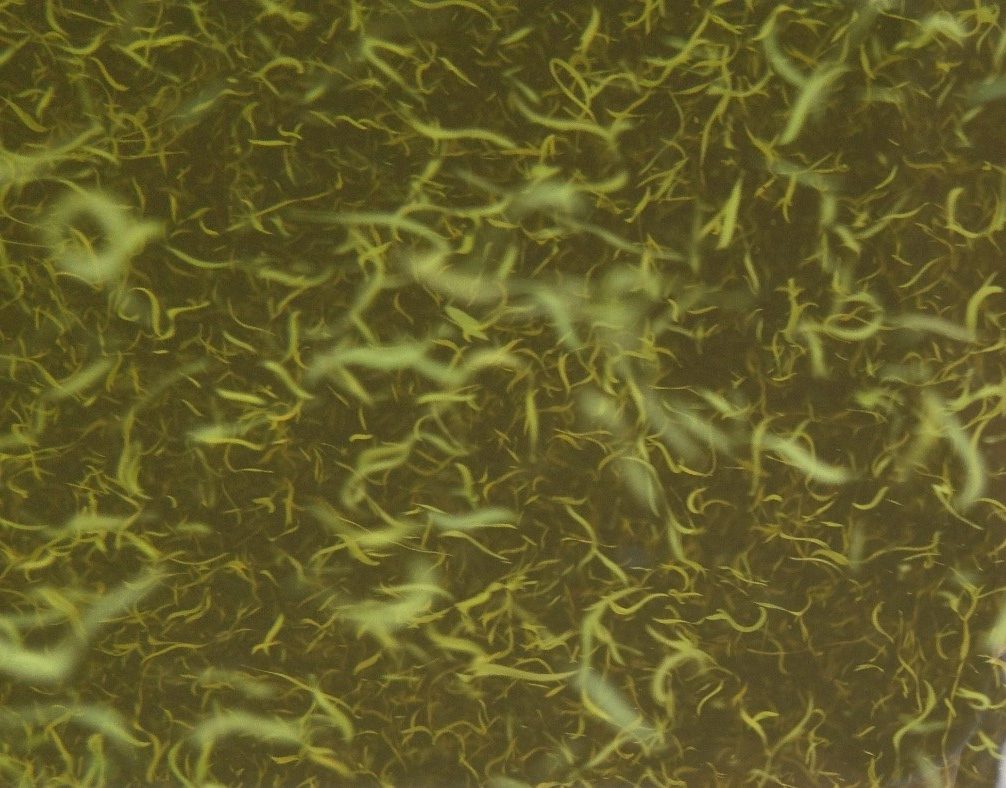
Figure A-6. Aphanizomenon flos-aquae, Klamath Lake, OR. Closer look at the “grass clippings.”
Source: Jacob Kann.

Figure A-7. Aphanizomenon flos-aquae, Klamath Lake, OR. Note the grass-clipping-like appearance.
Source: Jacob Kann.
Aphanizomenon flos-aquae – Description and Microscopic Images
Description: Filaments free-floating, joined into grass-clipping-like flakes; microscopic or macroscopic (up to 2 cm long) colonies with trichomes oriented in parallel; trichomes straight, cylindrical, always without firm sheaths; trichomes uniseriate, in the middle part usually cylindrical and isopolar, with distinctly elongated and, in older trichomes, vacuolized cells at the ends, with the apical cells long, cylindrical, and hyaline (at the end rounded or obtuse), with one to three distant, intercalary-developed heterocytes, which are localized subsymmetrically at the fully developed trichomes (shifted to one end of a trichome). Vegetative cells cylindrical or barrel-shaped, more or less isodiametrical or slightly shorter or longer than wide, pale blue-green or blue-green, usually with aerotopes. Heterocytes barrel-shaped or cylindrical with rounded or obtuse ends, which can completely disappear under special conditions (under high nitrogen content). Akinetes rarely spherical or widely oval, usually oval to long, cylindrical with rounded ends, developing paraheterocytically, solitarily, or in short rows (in two or three), close to heterocytes or slightly distant from them, usually in asymmetrical position in a trichome. Planktonic, sometimes forming characteristic water blooms, A. flos-aquae is important in managed fish ponds and in lakes; mainly are distributed in eutrophic systems.
Secondary Compounds: Cylindrospermopsin, saxitoxin, taste and odor compounds
Growth Habit: Forms scums in calm weather; can look like “grass flakes” in the water
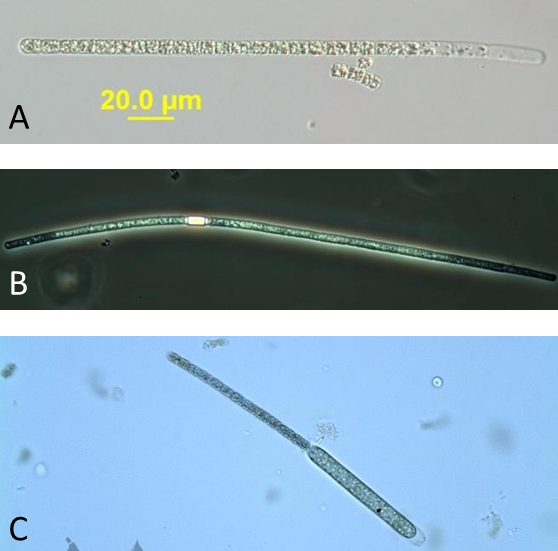
Figure A-8. Aphanizomenon flos-aquae. A: Nomarski, B: Phase, C: Brightfield.
Source: Barry Rosen (A) and Ann St. Amand (B and C).

Figure A-9. Aphanizomenon flos-aquae.
Source: Ann St. Amand.
Aphanizomenon gracile – Description and Microscopic Images
Description: Filaments free-floating, not joined into grass-clipping-like flakes; microscopic or macroscopic (up to 2 cm long) colonies with trichomes oriented in parallel; trichomes straight, cylindrical, always without firm sheaths; trichomes uniseriate, in the middle part usually cylindrical and isopolar, with distinctly elongated and, in older trichomes, vacuolized cells at the ends, with the apical cells long, cylindrical, and hyaline (at the end rounded or obtuse), with one to three distant, intercalary-developed heterocytes, which are localized subsymmetrically at the fully developed trichomes (shifted to one end of a trichome). Vegetative cells cylindrical or barrel-shaped, more or less isodiametrical or slightly shorter or longer than wide, pale blue-green or blue-green, usually with aerotopes. Heterocytes barrel-shaped or cylindrical with rounded or obtuse ends, which can completely disappear under special conditions (under high nitrogen content). Akinetes rarely spherical or widely oval, usually oval to long, cylindrical with rounded ends, developing paraheterocytically, solitarily, or in short rows (in two or three), close to heterocytes or slightly distant from them, usually in asymmetrical position in a trichome. Planktonic, sometimes forming characteristic water blooms.
Secondary Compounds: Cylindrospermopsin, saxitoxin, taste and odor compounds
Growth Habit: Does not form “grass flakes” in the water
Figure A-10. Aphanizomenon gracile, phase.
Source: Ann St. Amand.
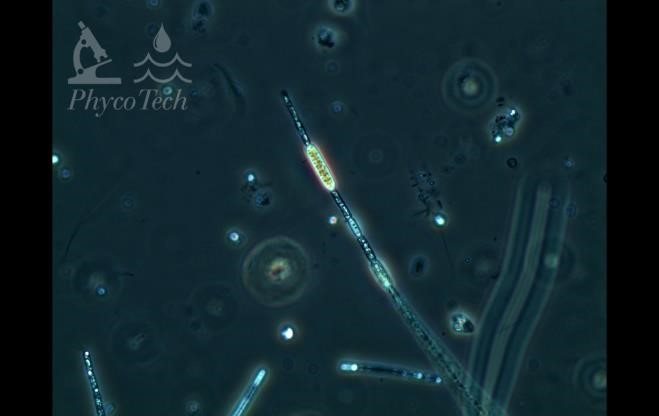
Figure A-11. Aphanizomenon gracile, phase.
Source: Ann St. Amand.
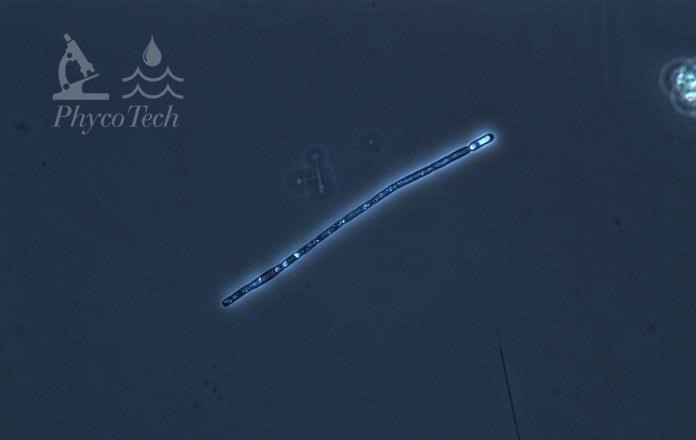
Figure A-12. Aphanizomenon gracile, phase.
Source: Ann St. Amand.
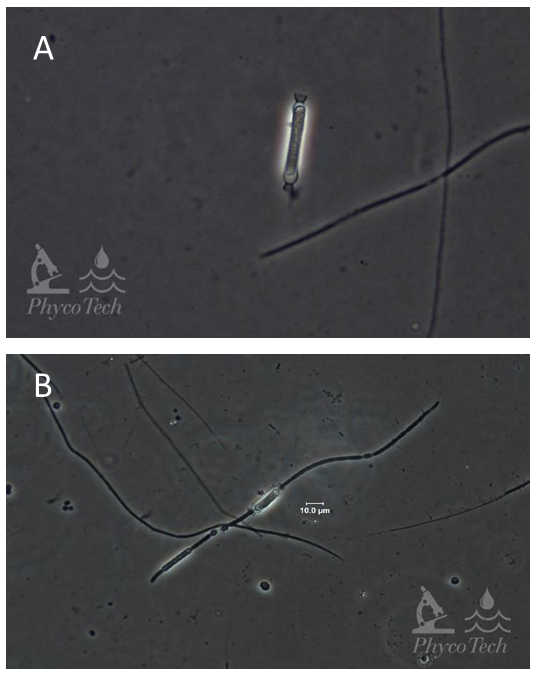
Figure A-13. Aphanizomenon gracile, akinete (A) and pro-akinete (B), phase.
Source: Ann St. Amand.
Dolichospermum (Anabaena) lemmermannii – Bloom Images

Figure A-14. Dolichospermum lemmermannii, Paw Paw Lake, MI.
Source: Ann St. Amand.

Figure A-15. Dolichospermum lemmermannii, Little Paw Paw Lake, MI.
Source: Ann St. Amand.

Figure A-16. Dolichospermum lemmermannii, Stony Lake, MI.
Source: Ann St. Amand.
Dolichospermum (Anabaena) lemmermannii – Description and Microscopic Images
Description: Gas vesicles occur obligatorily in cells in vegetative phase. They are joined into irregular aerotopes over the whole cell volume; aerotopes are recognizable in cells under optical microscope. Heterocytes arise intercalarly, solitarily. Akinetes develop paraheterocytically, with heterocytes adjacent to or between akinetes. Often forms the pattern of akinete-heterocyte-akinete. Akinetes often arise after fusion of two or few neighboring vegetative cells. Ripe akinetes are usually three or more times larger than vegetative cells. Planktic in vegetative state; never form sessile mats on the substrate. Filaments in small, contorted clusters, with akinetes clustered in center of colony. Protozoans (Vorticella spp.) often attached to colonies.
Secondary Compounds: Anatoxin-a, microcystin, saxitoxin, taste and odor compounds
Growth Habit: Forms scums in calm weather; can look like blue-green, cyan, or pink paint on the water surface or on substrates around the shoreline.

Figure A-17. Dolichospermum lemmermannii.
Source: Barry Rosen.
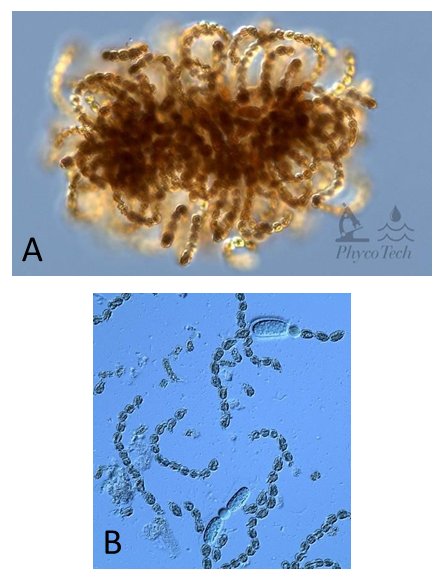
Figure A – 18. Dolichospermum lemmermannii, Nomarski. A – colony showing typical organization of akinetes surrounded by cells. B – colonies easily fragment into short chains of cells and akinetes.
Source: Ann St. Amand.
Dolichospermum (Anabaena) mendotae – Bloom Images

Figure A-19. Dolichospermum mendotae, Lake Monona, WI. Note the discolored water and the turquoise coloring that can look like spilled paint.
Source: Ann St. Amand.

Figure A-20. Dolichospermum mendotae, Lake Monona, WI.
Source: Ann St. Amand. Used with permission.

Figure A-21. Dolichospermum mendotae, Lake Monona, WI.
Source: Ann St. Amand. Used with permission.
Dolichospermum (Anabaena) mendotae – Description and Microscopic Images
Description: Gas vesicles occur obligatorily in cells in vegetative phase. They are joined into irregular aerotopes over the whole cell volume; aerotopes are recognizable in cells under optical microscope. Heterocytes arise intercalarly, solitarily. Akinetes develop paraheterocytically (in the middle of filaments), with heterocytes distant from (not next to) akinetes. Akinetes often arise after fusion of two or few neighboring vegetative cells. Ripe akinetes are usually three or more times larger than vegetative cells. Planktic in vegetative state; never form sessile mats on the substrate. Filaments in small, contorted clusters, with akinetes clustered in multiple places in colonies.
Secondary Compounds: Anatoxin-a, cylindrospermopsin, taste and odor compounds
Growth Habit: Forms scums in calm weather; can look like blue-green, cyan, or pink paint on the water surface or on substrates around the shoreline

Figure A-22. Dolichospermum mendotae.
Source: Ann St. Amand.
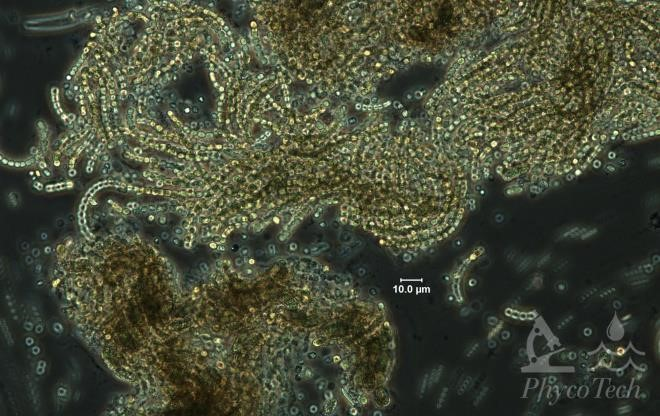
Figure A-23. Dolichospermum mendotae, phase.
Source: Ann St. Amand.
Gloeotrichia echinulata – Bloom Images

Figure A-25. Gloeotrichia echinulata, NH.
Source: Midge Eliassen.

Figure A-24. Gloeotrichia echinulata, NH.
Source: Midge Eliassen.

Figure A-26. Gloeotrichia echinulata, NH. This genus can be mistaken for pollen.
Source: Midge Eliassen.
Gloeotrichia echinulata – Description and Microscopic Images
Description: Filamentous-colonial; trichomes heteropolar with basal heterocytes and apical hair-like ends with own sheaths, united radially into gelatinous, hemispherical, or spherical colonies, which are microscopic up to several centimeters in diameter, olive-green, yellow-green, brown, or dark blue-blackish. The whole colony enveloped by a fine or firm slime; trichomes always oriented with heterocytes into the center of the colony. Trichomes often have a curved basal sheath that contains the akinete and persists in the sediments. Colonies are joined to the substrate or free-floating. Cells contain aerotopes. Often blooms in relatively nutrient-poor waters, compared with other scum-forming cyanobacteria.
Secondary Compounds: Microcystin, taste and odor compounds
Growth Habit: Forms scums in calm weather; often looks like small balls in the water column; often has a fuzzy or halo appearance when viewed in the water

Figure A-27. Gloeotrichia echinulata.
Source: Ann St. Amand.

Figure A-29. Gloeotrichia echinulata. Older colonies and grazed colonies will have shorter filaments.
Source: Ann St. Amand.
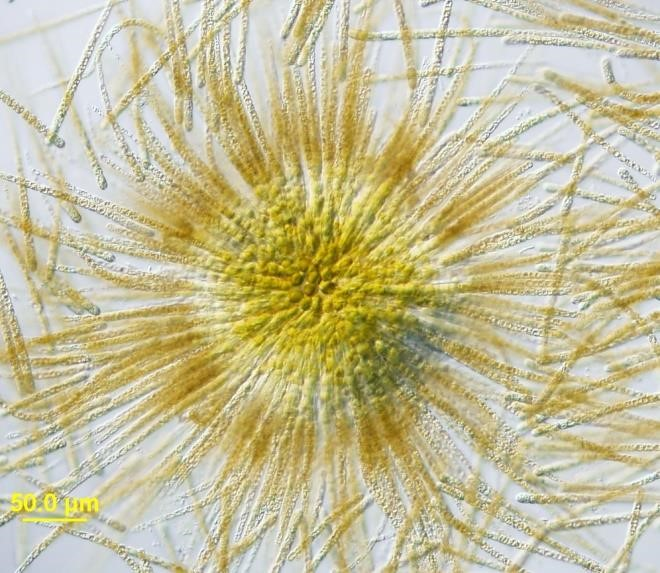
Figure A-28. Gloeotrichia echinulata, Nomarski.
Source: Barry Rosen.
Microseira (Lyngbya/Plectonema) wollei – Bloom Image

Figure A-30. Microseira wollei.
Source: Ken Wagner.
Microseira (Lyngbya/Plectonema) wollei – Description and Microscopic Images
Description: Filamentous; filaments long, isopolar, solitary, or in macroscopic clusters. Always with more or less thick, firm, colorless sheaths, lamellated, open at the apex, obligately and frequently false-branched. Trichomes isopolar, 16–25 µm wide, uniseriate, composed of short cylindrical or barrel-like (discoid) cells (always shorter than wide, usually several times), unconstricted at the crosswalls, not attenuated. Cells without aerotopes, sometimes with granular, blue-green, olive-green, or grey-blue content; end cells widely rounded, sometimes capitate or with slightly thickened outer cell wall. Heterocytes and akinetes absent. In clear (usually katharobic to oligosaprobic) water basins, springs, or creeks, in metaphyton and periphyton.
Secondary Compounds: Lyngbyatoxin, Lyngbya wollei Toxin-1 (LWTX-1)
Growth Habit: Mats float to surface in calm weather, forming huge mats on the lake surface that are difficult to navigate through; very difficult to control

Figure A-31. Microseira wollei, Nomarksi.
Source: Ann St. Amand.
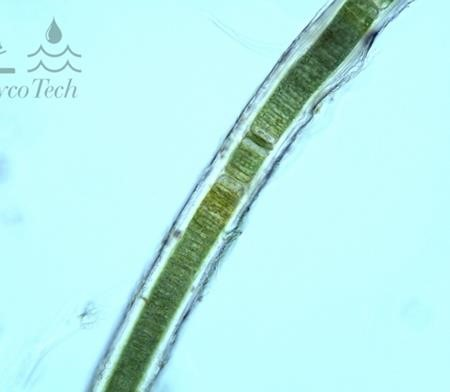
Figure A-32. Microseira wollei. Note the prominent sheath.
Source: Ann St. Amand.
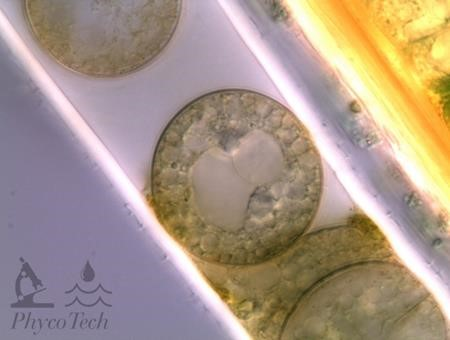
Figure A-33. Microseira wollei. Degrading trichome; cells may appear sideways instead of stacked. Nomarski.
Source: Ann St. Amand.
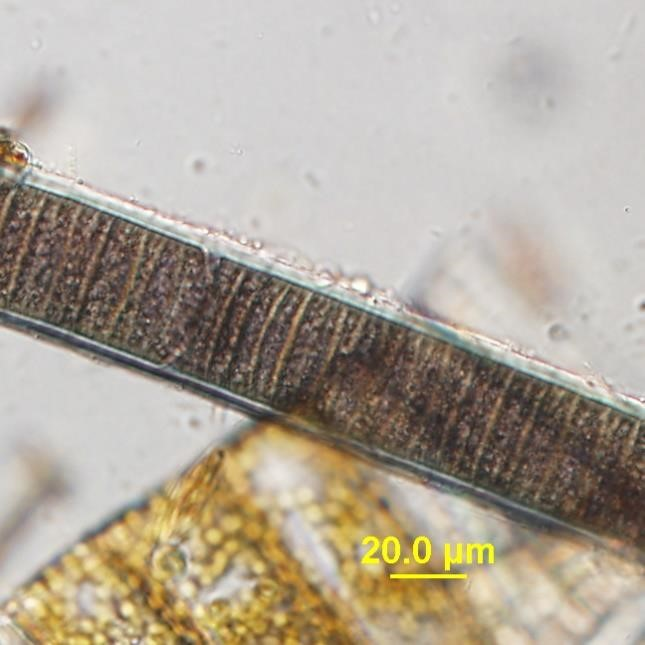
Figure A-34. Microseira wollei.
Source: Barry Rosen.
Microcystis aeruginosa – Bloom Images

Figure A-37. Microcystis aeruginosa, Orlando, FL.
Source: Ann St. Amand.

Figure A-36. Microcystis aeruginosa, FL.
Source: Andrew Chapman.

Figure A-35. Microcystis aeruginosa, Orlando, FL.
Source: Ann St. Amand.




Figure A-38. Microcystis aeruginosa, Central Park Lake, KS.
Source: Elizabeth Fabri Smith.




Figure A-39. Microcystis aeruginosa, Central Park Lake, KS.
Source: Elizabeth Fabri Smith.

Figure A-40. Microcystis aeruginosa, Marion Reservoir, KS. Note the blue coloring on the shore indicating dead and dying cells.
Source: Elizabeth Fabri Smith.

Figure A-41. Microcystis aeruginosa, Marion Reservoir, KS.
Source: Elizabeth Fabri Smith.




Figure A-42. Microcystis aeruginosa, Marion Reservoir, KS.
Source: Elizabeth Fabri Smith.

Figure A – 43. Microcystis aeruginosa.
Source: Jacob Kann.

Figure A-44. Microcystis aeruginosa, Marion Reservoir, KS.
Source: Elizabeth Fabri Smith.

Figure A-45. Microcystis aeruginosa, Marion Reservoir, KS. Note the variety of colors that can be present in a dense and aging scum.
Source: Elizabeth Fabri Smith
Microcystis aeruginosa – Description and Microscopic Images
Description: Cells spherical or (after division) hemispherical, with homogeneous, blue-green, greyish, or yellowish content. Cells have numerous aerotopes, especially in older colonies. Cells are typically imbedded in a gelatinous matrix and free-floating. Colonies can be large enough to be visible without a microscope and are amorphous, irregular, and sometimes net-like or clathrate. Quite common as free-floating in the plankton of freshwater and eutrophic lakes and reservoirs (often forming heavy water blooms). This genus is distinguished from Aphanocapsa by its ability to form aerotopes.
Secondary Compounds: Microcystin, taste and odor compounds
Growth Habit: Forms scums in calm weather; can look “chunky” in the water. There is some evidence that at least five species of Microcystis are all one species; however, we are treating them as separate species (Otsuka et al. 2001). Colonies often dissociate in Lugol’s iodine or as the bloom degrades, leaving numerous single cells.

Figure A-46. Microcystis aeruginosa. Colony shape is highly variable.
Source: Ann St. Amand.
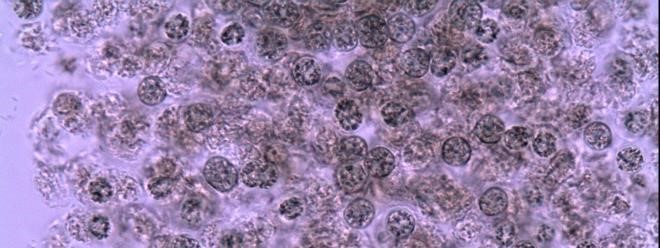
Figure A-48. Microcystis aeruginosa. Close-up of cells within a colony.
Source: Ann St. Amand.
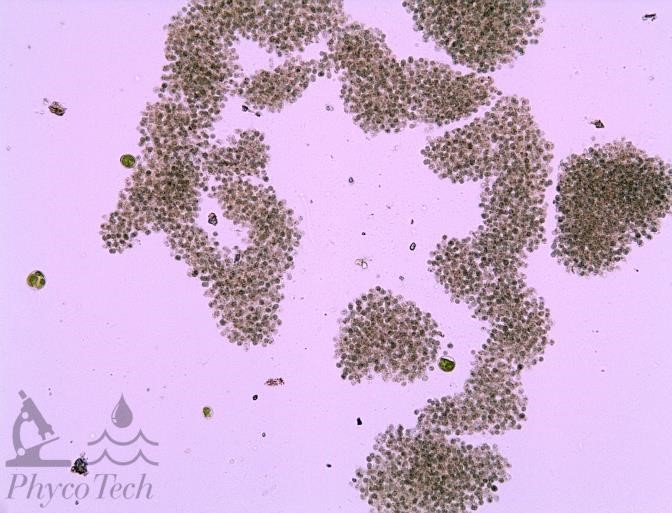
Figure A-47. Microcystis aeruginosa. Colonies may be lobed or contain holes.
Source: Ann St. Amand.
Microcystis viridis – Bloom Images

Figure A-49. Microcystis viridis, Paw Paw Lake, MI. Note the discolored water; not all HCBs form surface scums.
Source: Ann St. Amand.

Figure A-50. Microcystis viridis, Paw Paw Lake, MI.
Source: Ann St. Amand.

Figure A-51. Microcystis viridis, Paw Paw Lake, MI.
Source: Ann St. Amand.
Microcystis viridis – Description and Microscopic Images
Description: Cells spherical or (after division) hemispherical, with homogeneous, blue-green, greyish, or yellowish content. Cells have numerous aerotopes, especially in older colonies. Cells are typically imbedded in a gelatinous matrix in “packets” and free-floating. Colonies can be large enough to be visible without a microscope and are amorphous, irregular, and sometimes net-like. Free-floating in the plankton of freshwater and eutrophic lakes and reservoirs (often forming heavy water blooms). This genus is distinguished from Aphanocapsa spp. by its ability to form aerotopes. This genus is distinguished from M. aeruginosa by its cells’ subgroup or packet formation. There is some evidence that many Microcystis species are “ecotypes” and not true species.
Secondary Compounds: Microcystin, taste and odor compounds
Growth Habit: Forms scums in calm weather; can look “chunky” in the water or be evenly distributed in turbid circumstances. Often co-occurs with Limnoraphis spp.
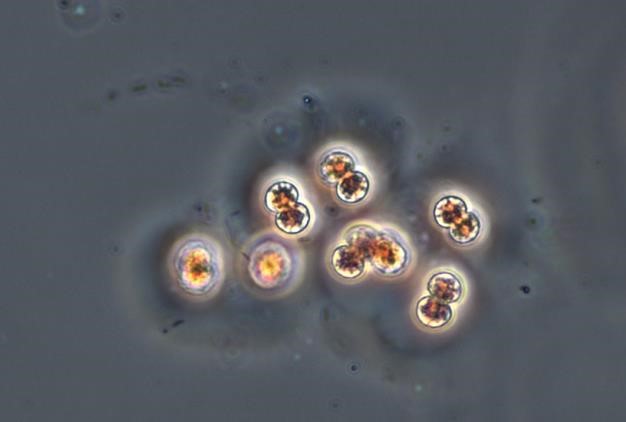
Figure A-52. Microcystis viridis, phase. This photo shows the colonial matrix.
Source: Ann St. Amand.
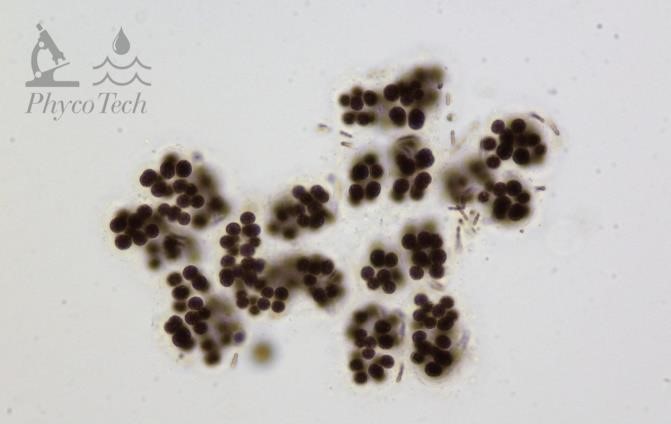
Figure A-53. Microcystis viridis.
Source: Ann St. Amand.
Nodularia spumigena – Bloom Images

Figure A-54. Nodularia spumigena, Great Salt Lake, UT. Note: We do not recommend handling HCBs without gloves.
Source: Wayne Wurtzbaugh.
Nodularia spumigena – Description and Microscopic Images
Description: Filamentous; filaments solitary or in groups or clusters, rarely in mats, isopolar, unbranched (without false or true branching), more or less straight, curved, coiled, or irregularly spirally coiled with fine, diffluent sheaths of a special two-layered structure (type species), opened at both ends. Trichomes uniseriate, cylindrical, rarely short, and slightly attenuated at the ends of developed trichomes, constricted at crosswalls, metameric, with more heterocytes in more or less regular distances from one another. Cells shortly barrel-shaped, their length never exceeds the width, with aerotopes. Cell content yellowish, pale olive-green, or blue-green, thylakoids irregularly coiled, spread all over the cell volume, sometimes more gathered in the peripheral layer of cells. Heterocytes of the same shape of cells, sometimes slightly differ in their size (slightly smaller or larger than vegetative cells). Akinetes shortly barrel-shaped (shorter than wide) or spherical, developing apoheterocytic, but often with irregularities. Cells divide crosswise to the trichome axis and grow to original size before the next division. All cells capable of division, without meristematic zones. Reproduction by hormogonia, dissociation of trichomes, and by akinetes. They occur rarely in freshwater reservoirs, but mainly in slightly saline or brackish waters and saline and coastal lakes.
Secondary Compounds: Nodularin, taste and odor compounds
Growth Habit: Forms scums in calm weather; forms huge mats on the lake surface that are difficult to navigate through

Figure A-55. Nodularia spumigena.
Source: Ann St. Amand.
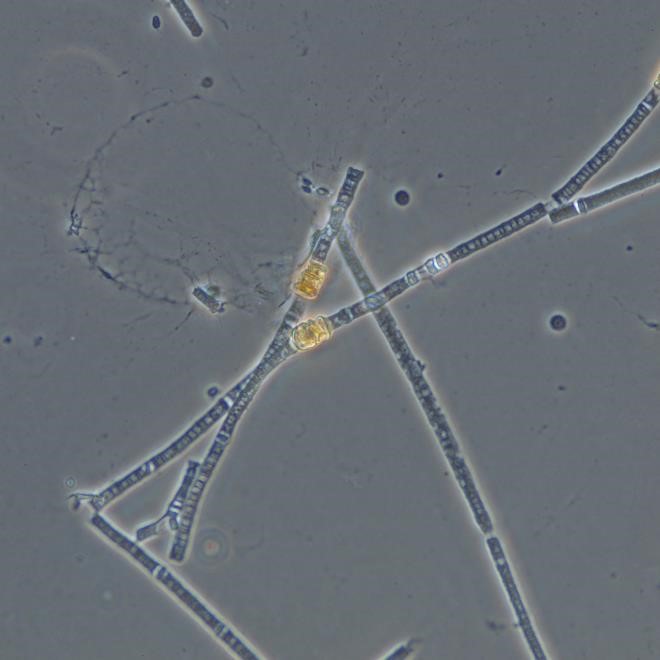
Figure A-56. Nodularia spumigena, phase.
Source: Ann St. Amand.
Planktothrix (Oscillatoria) agardhii/prolifica – Bloom Images

Figure A-57. Planktothrix agardhii subsp. rubescens/P. prolifica, Fleeinghorse Lake, Alberta, Canada. This taxon often appears pinkish-red.
Source: Ron Zurawell.

Figure A-58. Planktothrix agardhii subsp. rubescens/P. prolifica encased in ice, Fork Lake, Canada. This genus is commonly reported during the winter.
Source: Ron Zurawell.

Figure A-59. Planktothrix agardhii subsp. rubescens/P. prolifica, Fleeinghorse Lake, Alberta, Canada.
Source: Ron Zurawell.




Figure A-60. Planktothrix agardhii subsp rubescens, Cedar Lake, MN. This taxon may occur in many shades of red.
Source: Rachel Crabb, Minneapolis Park & Recreation Board.

Figure A-61. Planktothrix agardhii subsp rubescens, Clear Lake, MN. This taxon often forms dispersed subsurface blooms.
Source: Rachel Crabb, Minneapolis Park & Recreation Board.

Figure A-62. Planktothrix agardhii subsp rubescens, Lake Nokomis, MN.
Source: Rachel Crabb, Minneapolis Park & Recreation Board.
Planktothrix (Oscillatoria) agardhii/prolifica – Description and Microscopic Images
Description: Filaments solitary, rarely in small, irregular, and easy-disintegrating fascicles (groups), more or less straight or slightly waved, isopolar, free-living; usually growing without sheath. Trichomes with cylindrical cells, slightly constricted at the crosswalls, sometimes slightly tapering to the ends, to 4 mm long, 3.5–10 µm wide, occasionally with slight movement (trembling). Cells slightly shorter than wide to almost isodiametric, rarely slightly longer than wide, with aerotopes through the whole cell volume (but sometimes without gas vesicles in parts of trichomes); end cells (when fully developed) widely rounded or slightly narrowed and with thickened outer cell wall or with calyptra. Heterocytes and akinetes absent. Mainly in mesotrophic to eutrophic reservoirs (usually lakes), often growing at depth. Can form red blooms under the ice in winter.
Secondary Compounds: Anabaenopeptin, microcystin
Growth Habit: Does not tend to form scums. Colors ice red in winter blooms. It is unclear if the red winter/spring blooms of Planktothrix are P.prolifica or P.agardhii subsp. rubescens; however, both species produce microcystin.
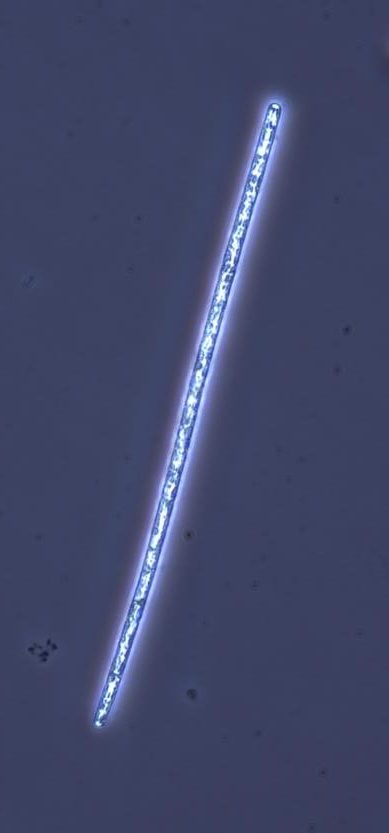
Figure A-63. Planktothrix agardhii subsp. rubescens, phase.
Source: Ann St. Amand.

Figure A-64. Planktothrix agardhii subsp. rubescens,
Source: Barry Rosen.
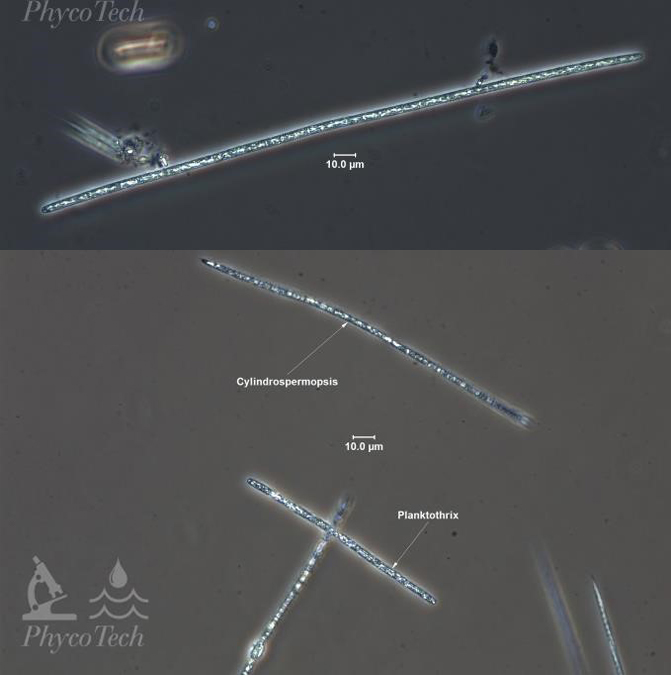
Figure A-65. Planktothrix agardhii subsp. rubescens, phase.
Source: Ann St. Amand.

Figure A-66. Planktothrix agardhii subsp rubescens. Note that a bloom may contain more than one species of cyanobacteria.
Source: Ann St. Amand.
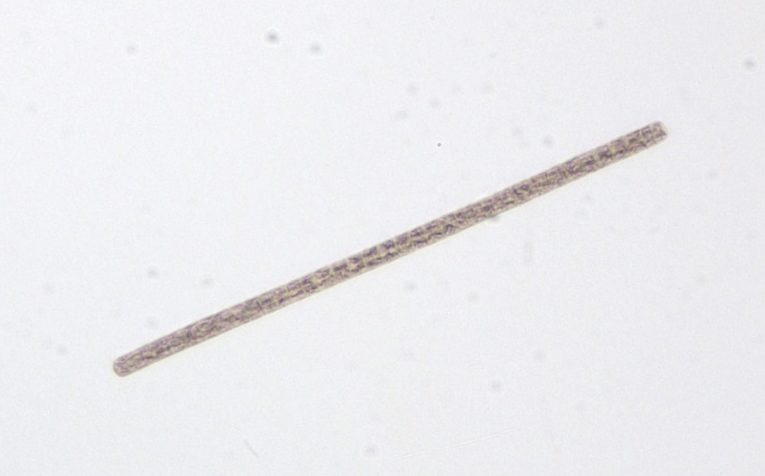
Figure A-67. Planktothrix agardhii subsp. rubescens.
Source: Ann St. Amand.
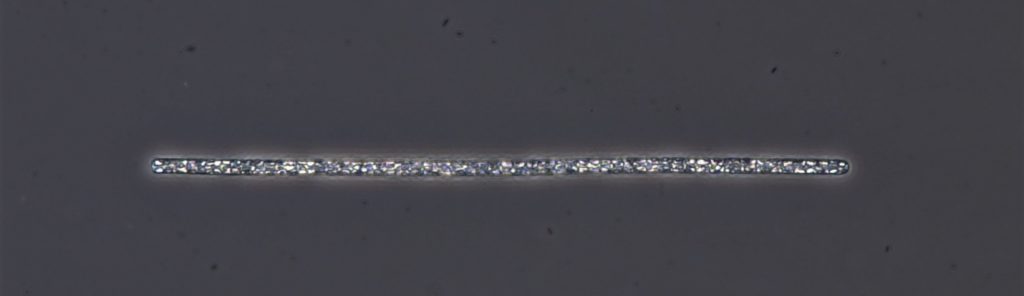
Figure A-68. Planktothrix agardhii subsp. rubescens.
Source: Ann St. Amand.
Raphidiopsis (Cylindrospermopsis) raciborskii – Bloom Images

Figure A-69. Raphidiopsis raciborskii, OK. This taxon does not form scums.
Source: Ann St. Amand.

Figure A-70. Raphidiopsis raciborskii, OK.
Source: Ann St. Amand.

Figure A-71. Raphidiopsis raciborskii, IN.
Source: Michael Martin.
Raphidiopsis (Cylindrospermopsis) raciborskii – Description and Microscopic Images
Description: Filaments solitary, straight, or screw-like coiled and free-floating, slightly narrowed to both ends, without sheaths; trichomes isopolar. Cells cylindrical, isodiametric, or shorter or longer than wide, pale blue-green or yellowish, with aerotopes; end cells (before heterocyte formation) narrowed, conical, and bluntly pointed. Heterocytes ovoid or conical, sometimes slightly curved, unipored, and terminal; akinetes oval to cylindrical with rounded ends, developing asymmetrically on a trichome, solitary or in short rows (up to three), slightly distant from the terminal heterocytes, rarely close to heterocytes. Cells divide crosswise (sometimes asymmetrically) and grow more or less into the original size before the next division. Planktonic in eutrophic aquatic basin but does not form scums. Water is often an olive-brown color. R. raciborskii is a late summer bloomer and tends to be associated with a lack of zooplankton in the water column.
Secondary Compounds: Anatoxin-a, cylindrospermopsin, saxitoxin
Growth Habit: Does not form scums in calm weather.
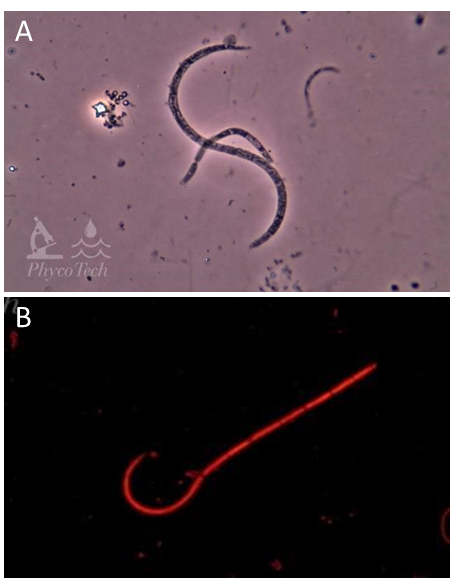
Figure A-72. Raphidiopsis raciborskii. Curled and curled to straight morphs. A: Phase, B: Epifluoresence, green excitation.
Source: Ann St. Amand.

Figure A-74. Raphidiopsis raciborskii. Straight morph, Nomarski.
Source: Barry Rosen.
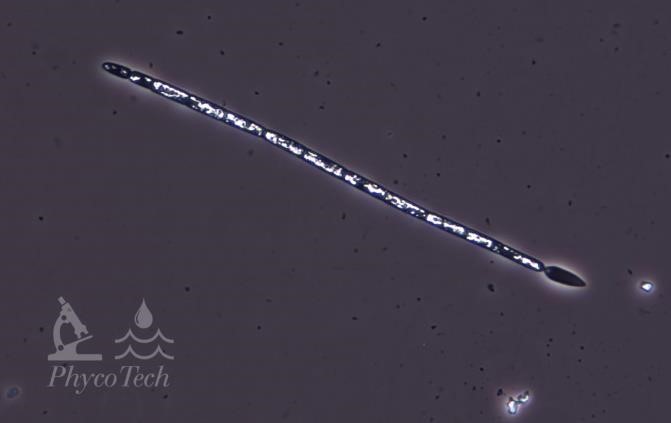
Figure A-73. Raphidiopsis raciborskii. Straight morph, phase. Note the teardrop-shaped cell at one end, a key characteristic.
Source: Ann St. Amand.
Woronichinia (Coelosphaerium) naegeliana – Bloom Images

Figure A-75. Woronichinia naegeliana, Kalamazoo County, MI.
Source: Ann St. Amand.

Figure A-76. Woronichinia naegeliana, Kalamazoo County, MI.
Source: Ann St. Amand.

Figure A-77. Woronichinia naegeliana.
Source: Linda Green, University of Rhode Island.
Woronichinia (Coelosphaerium) naegeliana – Description and Microscopic Images
Description: Colonial; colonies microscopic, spherical to irregularly oval, usually composed from subcolonies, free living (plankton, metaphyton), with narrow enveloping mucilaginous layer. Within colonies is a system of parallel and radially oriented tube-like stalks, +/- originating from the center of a colony (difficult to see in healthy colonies). Cells radially elongated, joined to the ends of stalks, widely oval or slightly obovoid, in old colonies usually very densely, radially agglomerated in the peripheral layer. Cells often appear as “doubles.” Cells pale blue-green or yellowish, with aerotopes.
Secondary Compounds: Microcystin, taste and odor compounds
Growth Habit: Forms scums in calm weather. Colonies often look like “gumdrops” under the microscope. Colonies often dissociate in Lugol’s iodine or as the bloom degrades, often leaving numerous single cells or doublets of cells.
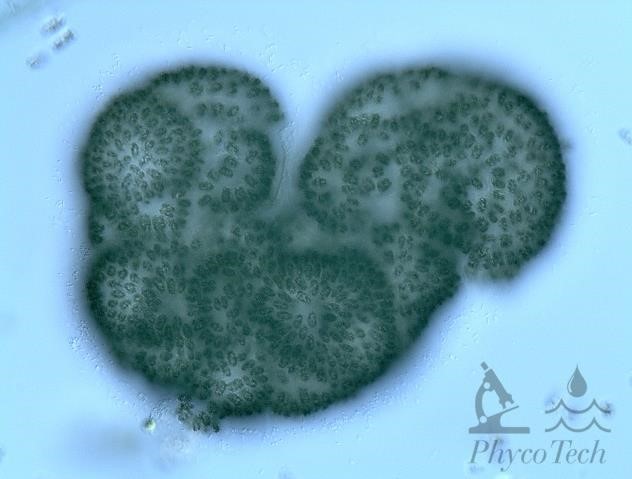
Figure A-78. Woronichinia naegeliana.
Source: Ann St. Amand.
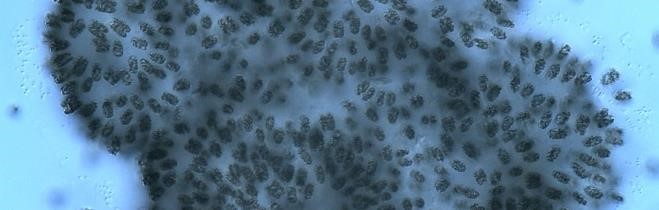
Figure A-79.Woronichinia naegeliana.
Source: Ann St. Amand.

Figure A-80. Woronichinia naegeliana, Nomarski.
Source: Barry Rosen.
A-6. Planktonic Cyanobacteria That Form Mixed-Assemblage Blooms – Field Images and Microscopic Images
The taxa presented in this section tend to appear in assemblages of mixed taxa and not as single taxon blooms.
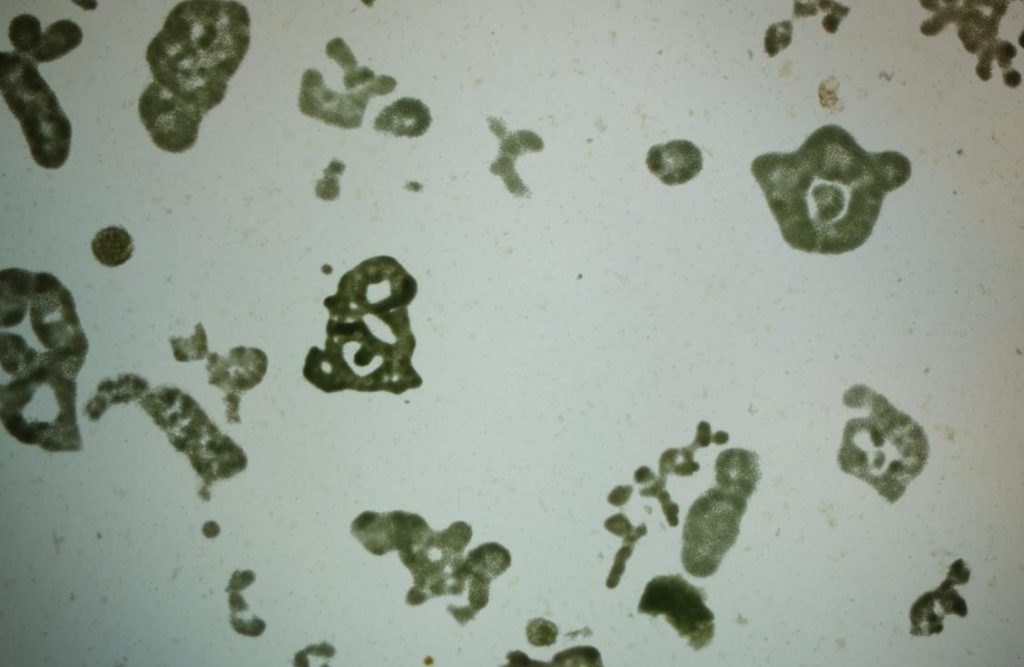
Figure A-81. Microcystis aeruginosa under low power showing various colony morphologies.
Source: Barry Rosen.

Figure A-82. Microcystis aeruginosa, Planktothrix agardhii, and Dolichospermum sp., epifluorescence green excitation.
Source: Ann St. Amand.
Cuspidothrix (Aphanizomenon) issatschenkoi
Description: Trichomes straight, with “telescopic” or stepped-down end cells on at least one end of the trichome. Cells not as wide as A. flos-aquae, with aerotopes that are sparse in the cells and often not obvious. Heterocytes are cylindrical. Akinetes are long and cylindrical with rounded ends.
Secondary Compounds: Anatoxin-a, saxitoxin
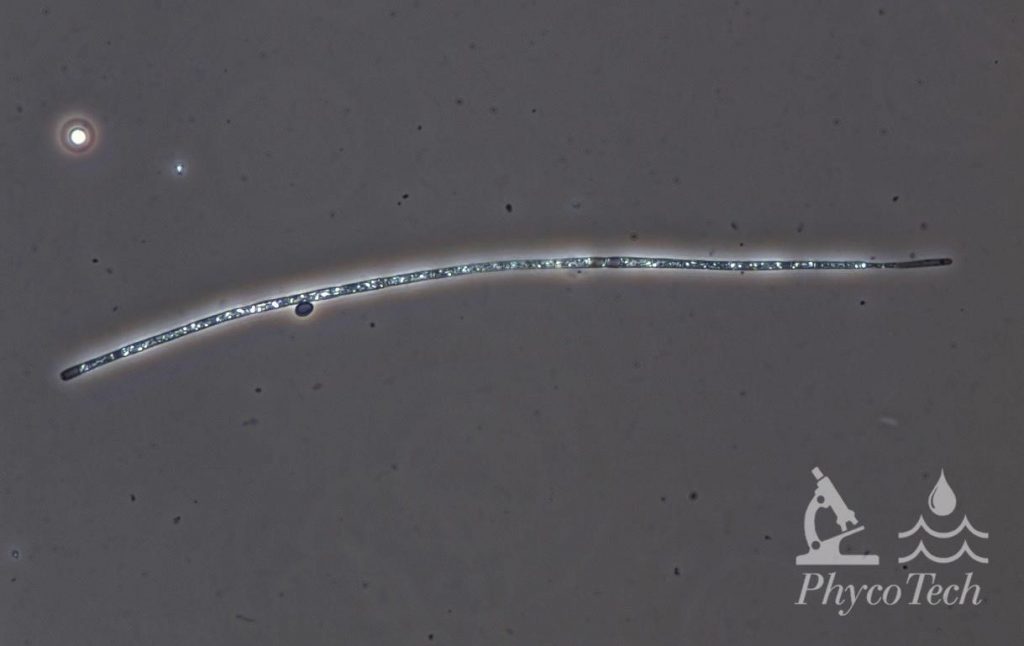
Figure A-83. Cuspidothrix issatschenkoi, phase.
Source: Ann St. Amand.
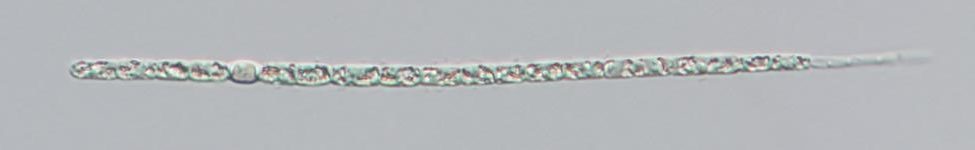
Figure A-84. Cuspidothrix issatschenkoi, Nomarski.
Source: Barry Rosen.
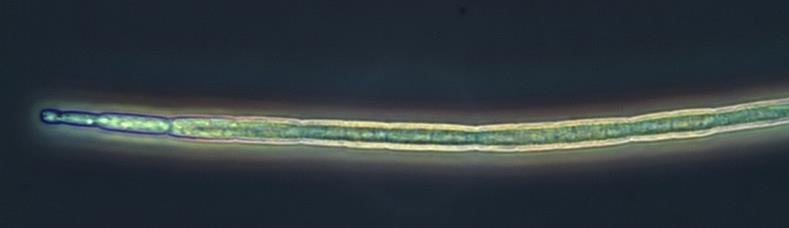
Figure A-85. Cuspidothrix issatschenkoi, phase.
Source: Ann St. Amand.
Chrysosporum (Aphanizomenon) ovalisporum
Description: Trichomes straight, with slightly elongated, attenuated end cells on at least one end of the trichome. Trichomes can be up to 1.5 mm long. Cells are 3–5 µm wide, slightly constricted, and three times longer than wide. Heterocytes round to ellipsoidal and quite distant from the oval akinetes, which gives the trichome a “striped” appearance.
Secondary Compounds: Cylindrospermopsin
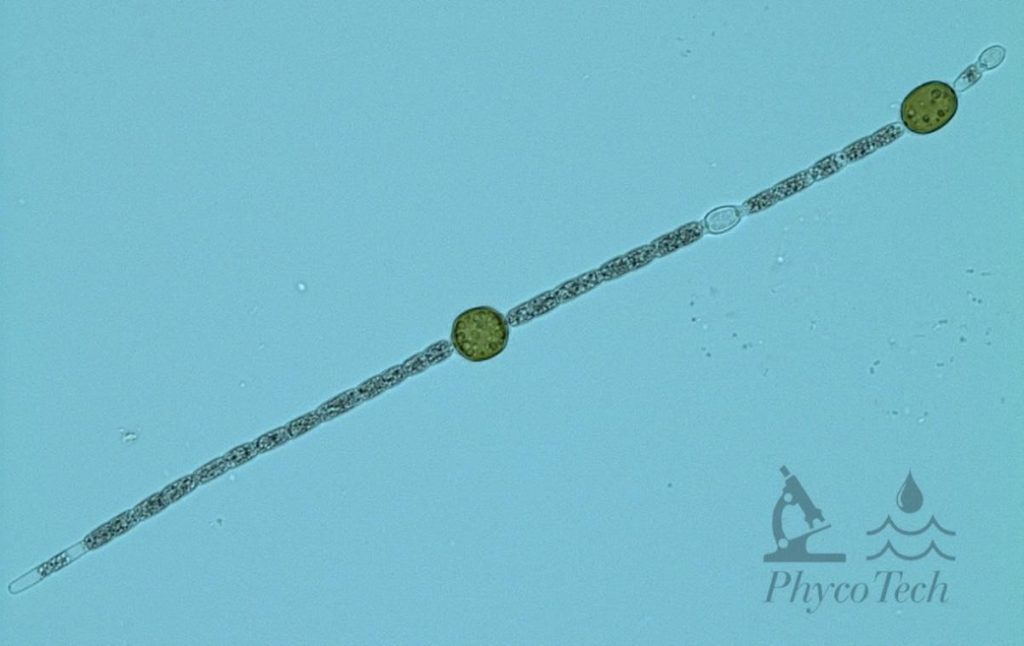
Figure A-86. Chrysosporum ovalisporum. Large, dark cells are akinetes; smaller, translucent cells are heterocytes.
Source: Ann St. Amand.

Figure A-87. Chrysosporum ovalisporum, Nomarski.
Source: Ann St. Amand.
Dolichospermum (Anabaena) circinale
Description: Twisted trichomes, often forming regular circular loops or loose, screw-like formations. Cells 5–11 µm wide, commonly 6–8 µm wide. Cells are constricted, about as long as wide, with aerotopes. Ellipsoidal or slightly asymmetrically shaped akinetes distant from the spherical heterocytes.
Secondary Compounds: Anatoxin-a, microcystin, saxitoxin
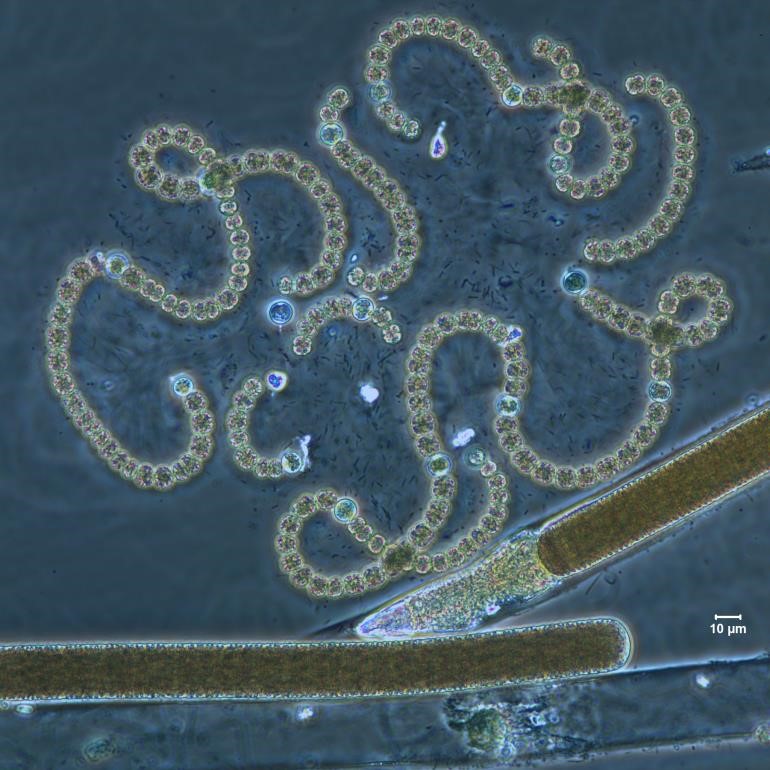
Figure A-88. Dolichospermum circinale. Note the curly, beads-on-a-string appearance. The straight trichome is Limnoraphis sp., phase.
Source: Ann St. Amand.
Figure A-89. Dolichospermum circinale, phase.
Source: Ann St. Amand
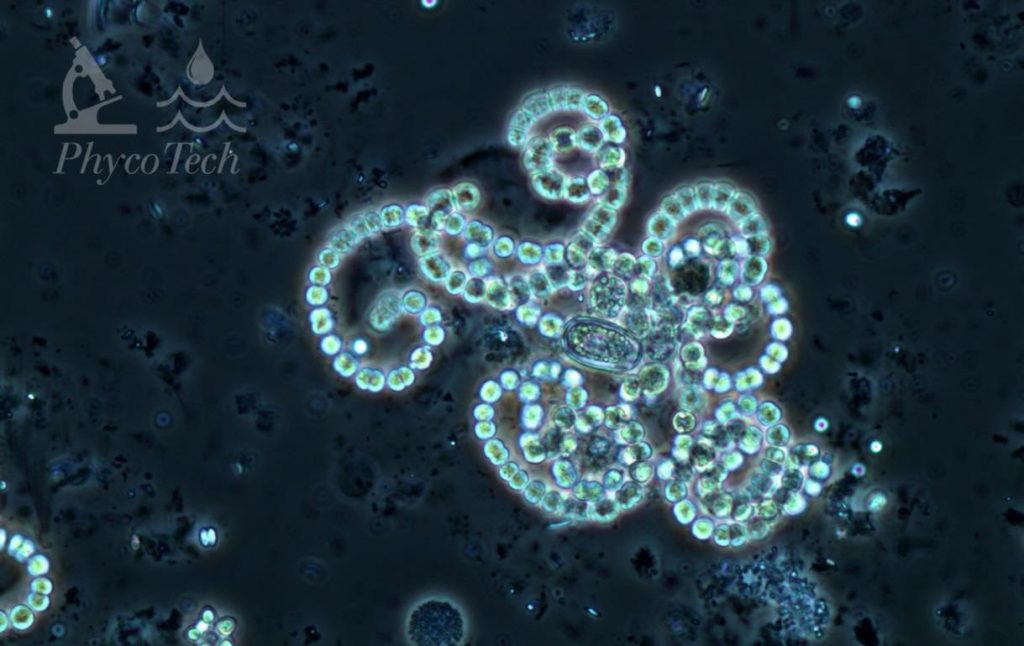
Dolichospermum (Anabaena) crassum
Description: Trichomes almost always forming regular coils in screw-like formations. Cells 5–12 µm wide, tending toward the larger size. Cells are constricted, about as long as wide, with aerotopes. Ovoid or cylindrical akinetes distant from the spherical heterocytes. Often found with larger cells than D. circinale.
Secondary Compounds: Anatoxin-a
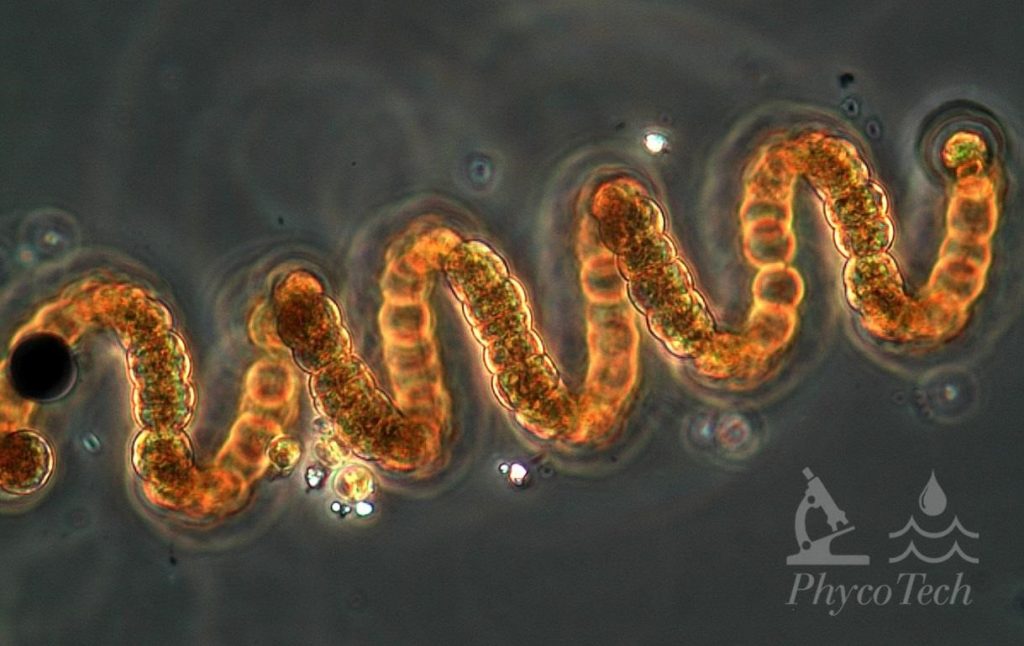
Figure A-90. Dolichospermum crassum, phase. Note the regular, corkscrew appearance.
Source: Ann St. Amand.
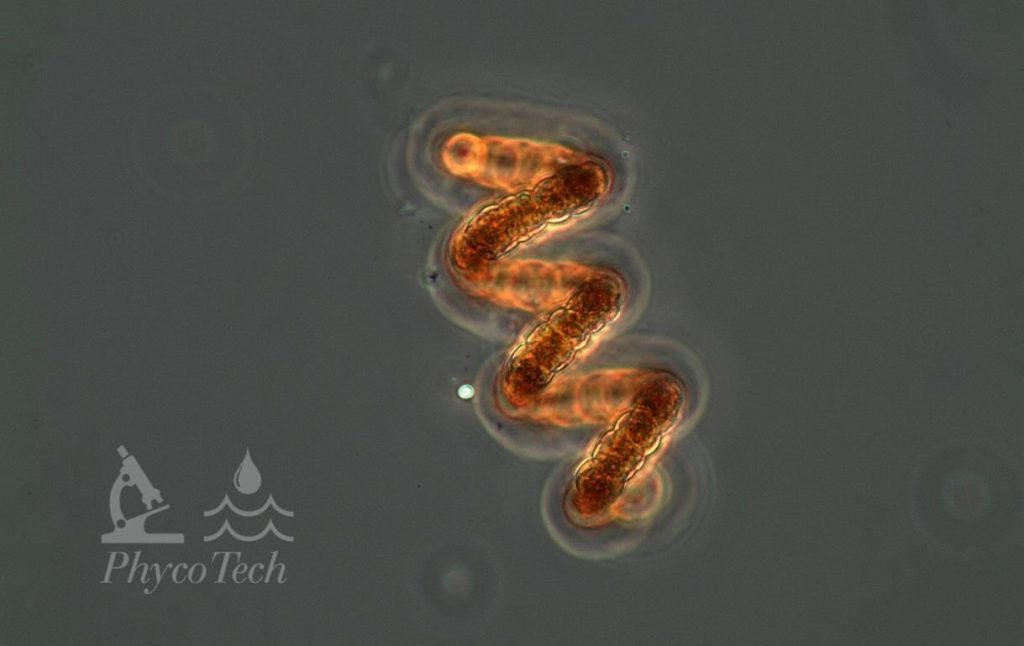
Figure A-91. Dolichospermum crassum, phase.
Source: Ann St. Amand.
Dolichospermum (Anabaena) planctonicum
Description: Straight trichomes, not attenuated. Cells 4–10 µm wide, tending toward the larger size. Cells are constricted, about as long as wide, with aerotopes. Oval to long, oval-shaped akinetes distant from the spherical heterocytes.
Secondary Compounds: Microcystin, saxitoxin
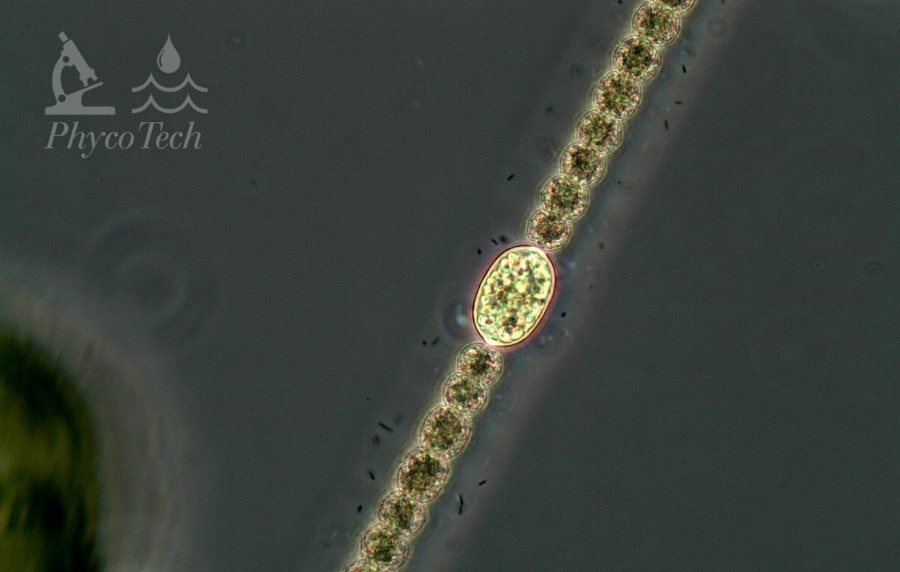
Figure A-92. Dolichospermum planctonicum. Note the prominent akinete, phase.
Source: Ann St. Amand.
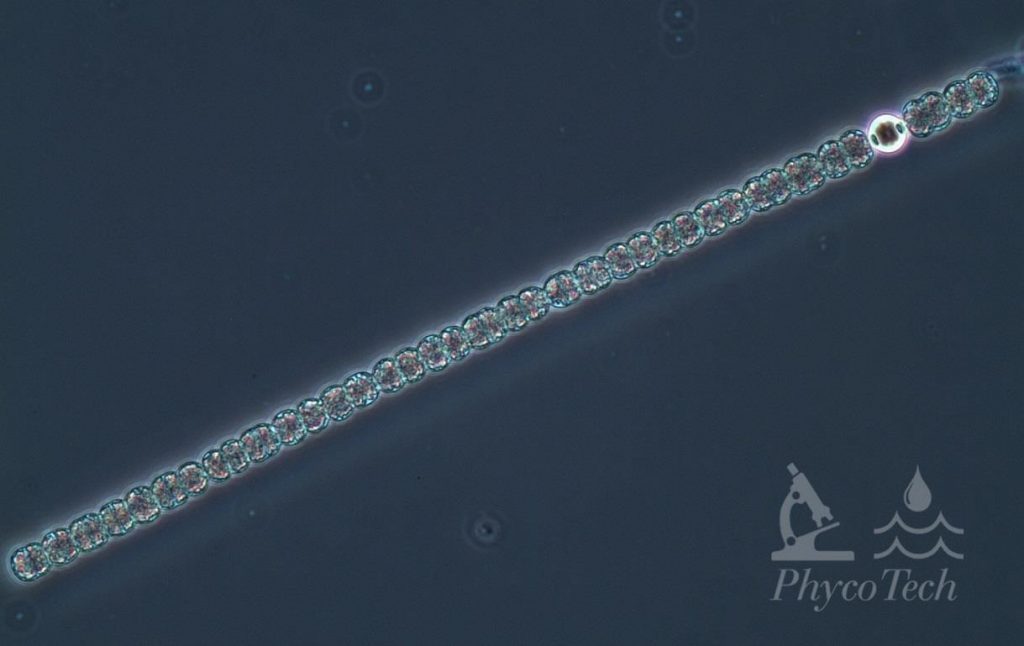
Figure A-93. Dolichospermum planctonicum, phase. Note the heterocyte.
Source: Ann St. Amand.
Limnoraphis (Lyngbya) birgei
Description: Trichome about 16 µm wide, filament about 20 µm wide. Cells 4 µm long, giving the filament a “stack of coins” appearance. Trichomes often without a sheath in the bloom, especially late in the season. Cells have aerotopes, so filaments and naked trichomes are buoyant. Often co-occurs with Microcystis and Dolichospermum.
Secondary Compounds: Suspected, but none currently confirmed
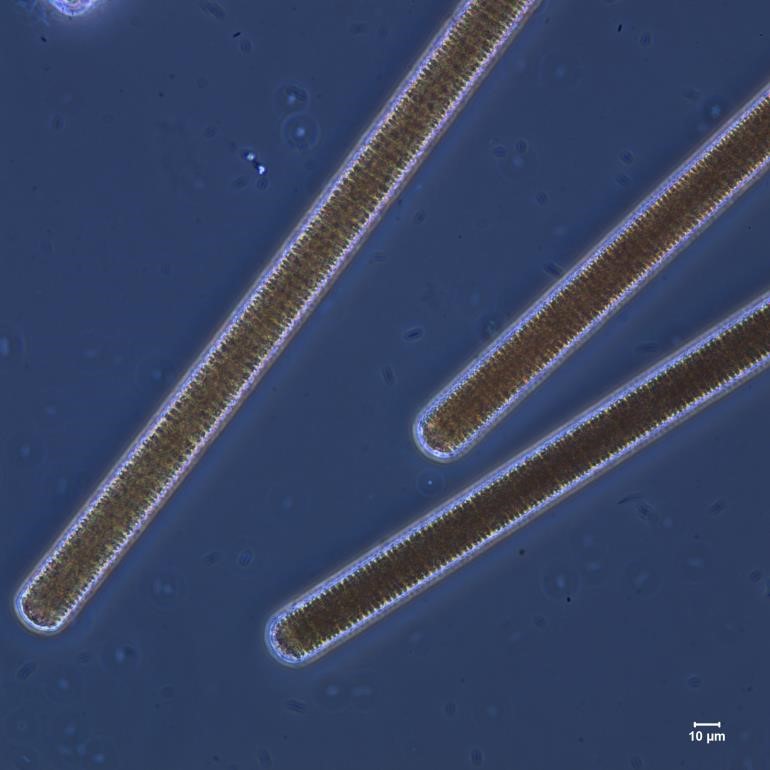
Figure A-94. Limnoraphis birgei, phase.
Source: Ann St. Amand.

Figure A-95. Limnoraphis birgei.
Source: Ann St. Amand.
Microcystis wesenbergii
Description: Cells often larger than M. aeruginosa and loosely held in a very robust, thick, and refractive sheath. Much rarer than M. aeruginosa. There is some evidence that many Microcystis species are “ecotypes” and not true species.
Secondary Compounds: Microcystin, anatoxin-a
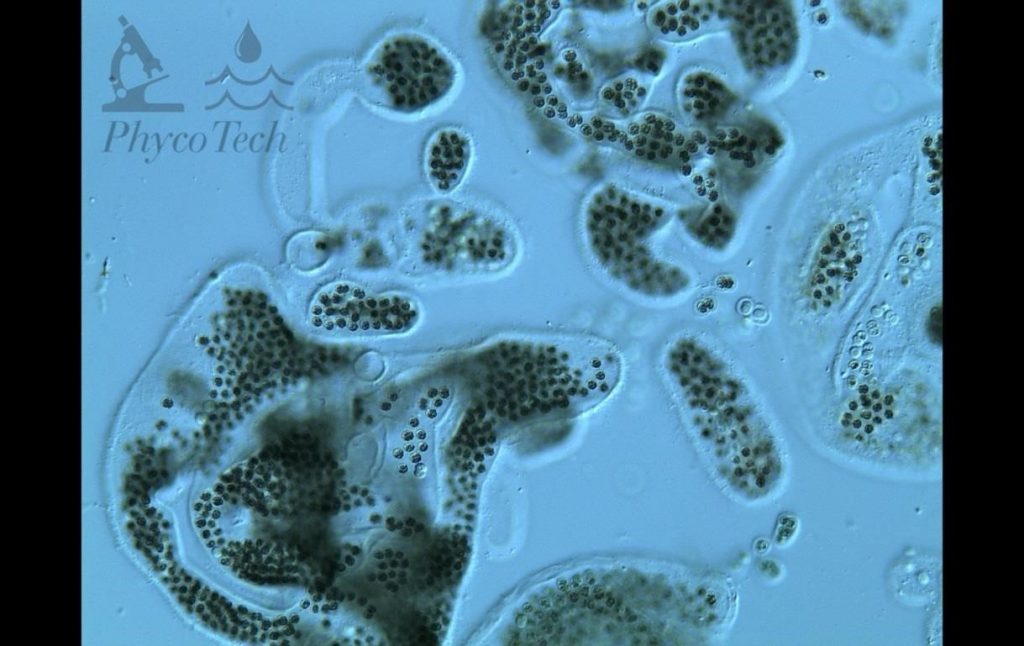
Figure A-96. Microcystis wesenbergii, Nomarski. Note the prominent colonial matrix.
Source: Ann St. Amand.
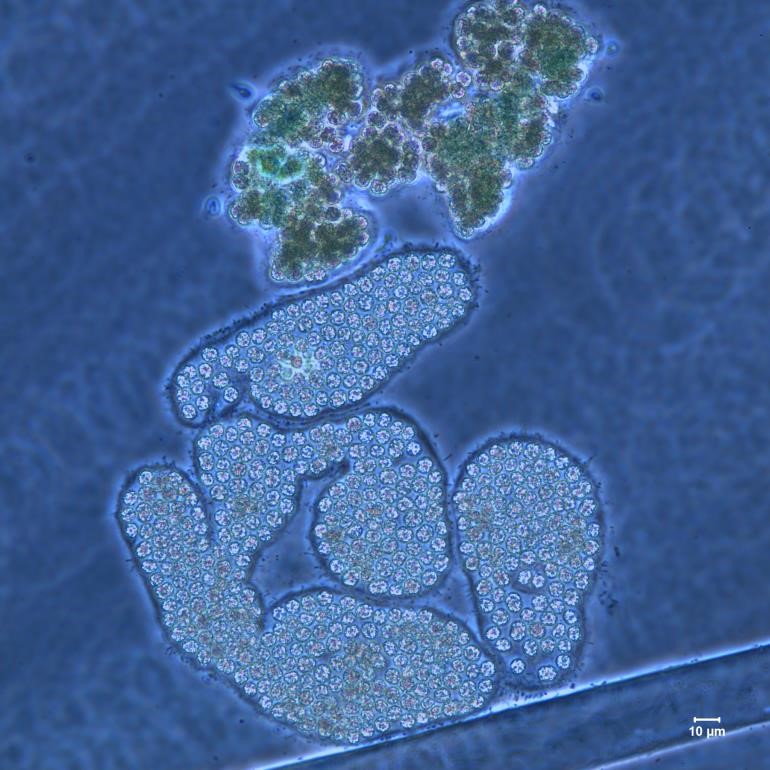
Figure A-97. Microcystis wesenbergii, M. viridis, Nomarski.
Source: Ann St. Amand.
Sphaerospermopsis (Aphanizomenon) aphanizomenoides
Description: Mostly straight trichomes, slightly attenuated end cells. Cells 5–7 µm wide. Cells are slightly constricted, longer than wide, with aerotopes. Clear pattern with rounded akinetes on one or both sides of the spherical or slightly elongated heterocytes.
Secondary Compounds: Anatoxin-a, microcystin
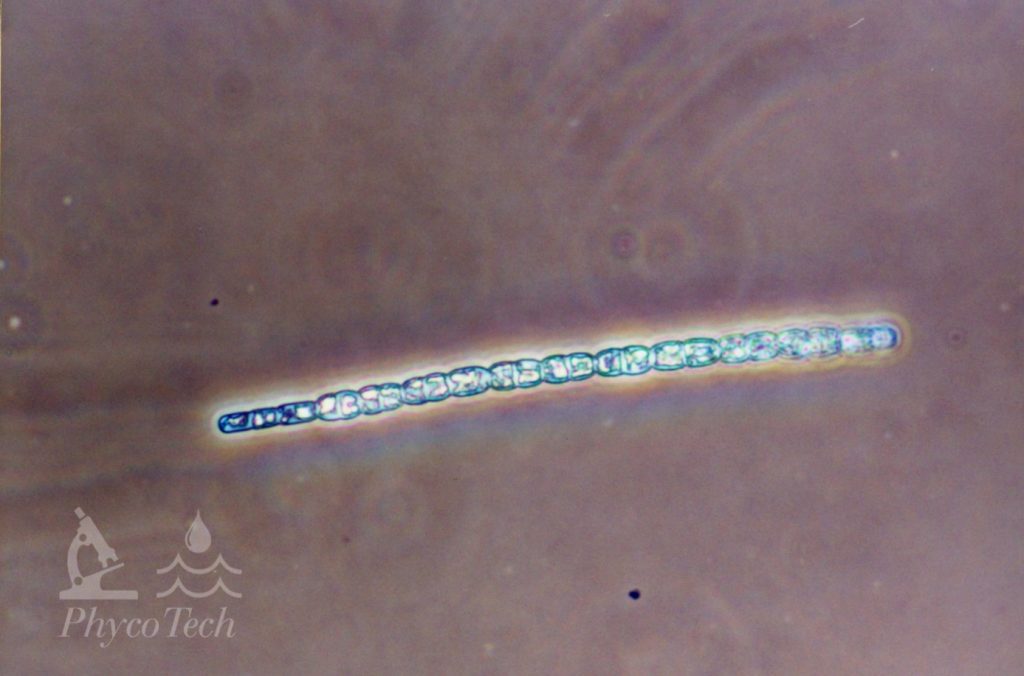
Figure A-98. Sphaerospermopsis aphanizomenoides. Note the attenuated cells on the left side.
Source: Ann St. Amand.

Figure A-99. Sphaerospermopsis aphanizomenoides.
Source: Ann St. Amand.
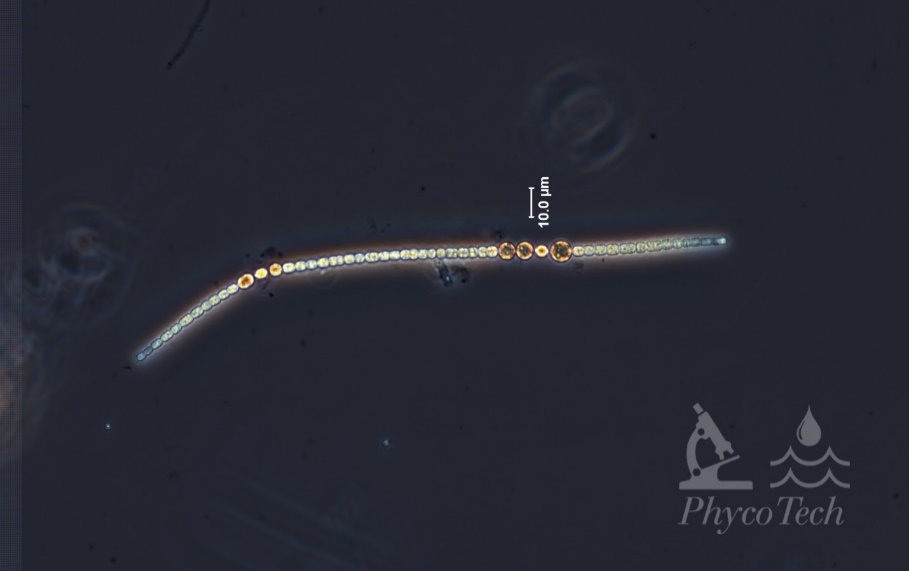
Figure A-100. Sphaerospermopsis aphanizomenoides. Note the position of akinetes and heterocytes.
Source: Ann St. Amand.
Sphaerospermopsis (Anabaena) torques-reginae
Description: Twisted trichomes, often forming circular loops or loose, screw-like formations. Cells 5–7 µm wide. Cells are constricted, about as long as wide, with aerotopes. Clear pattern with rounded akinetes on one or both sides of the spherical heterocytes.
Secondary Compounds: Microcystin
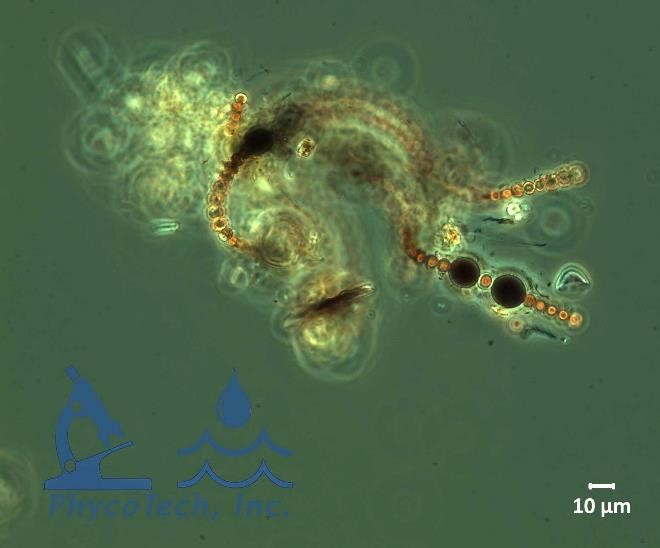
Figure A-101. Sphaerospermopsis torques-reginae, phase.
Source: Ann St. Amand.
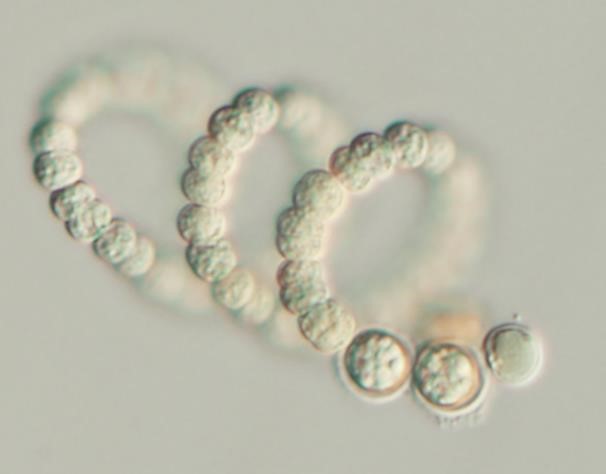
Figure A-102. Sphaerospermopsis torques-reginae, Nomarski.
Source: Barry Rosen.
Trichodesmium lacustre
Description: No heterocytes or akinetes; parallel trichomes in a colony or fascicle. Cells about as long as wide, constricted and with aerotopes, 5–6 µm wide. End cells sometimes lengthened.
Secondary Compounds: Microcystin, saxitoxin
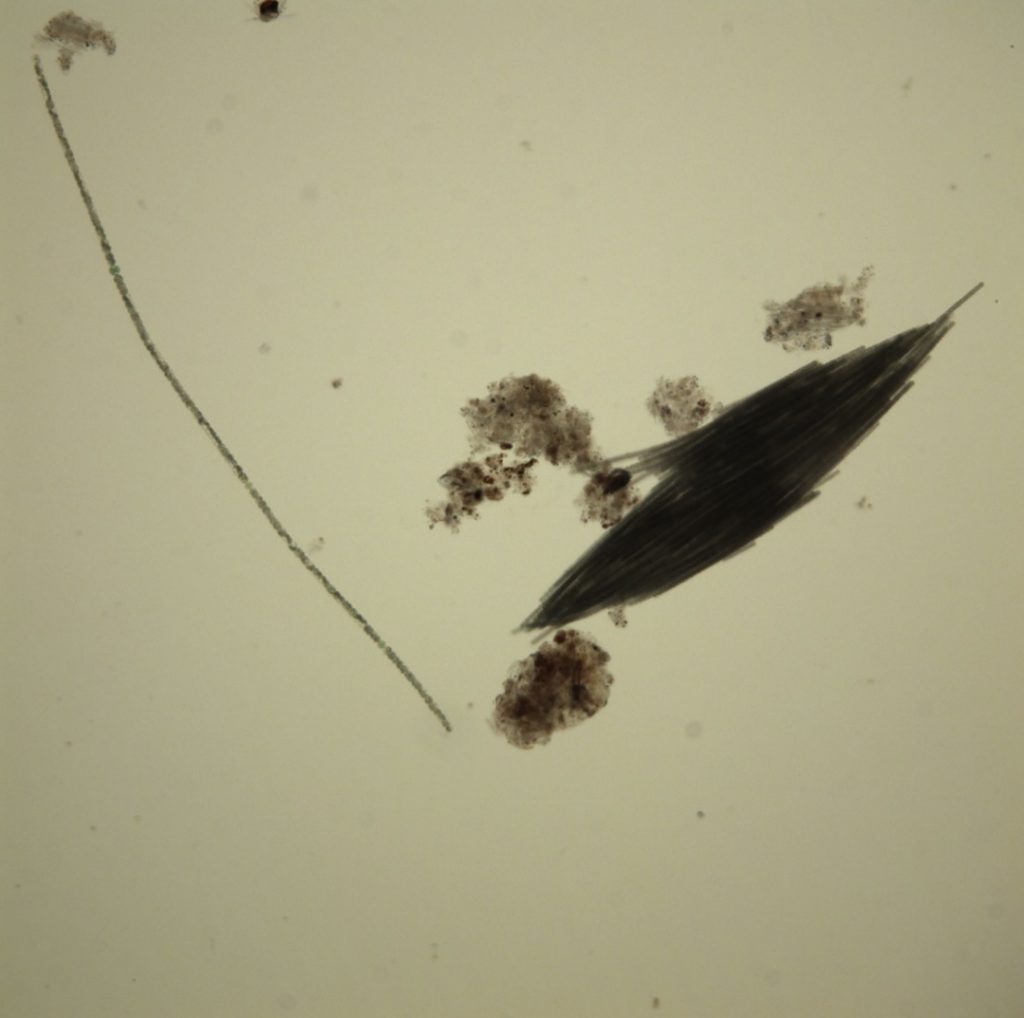
Figure A-103. Trichodesmium lacustre. Note the bundled trichomes, which gives it a “flaky” appearance.
Source: Ann St. Amand.
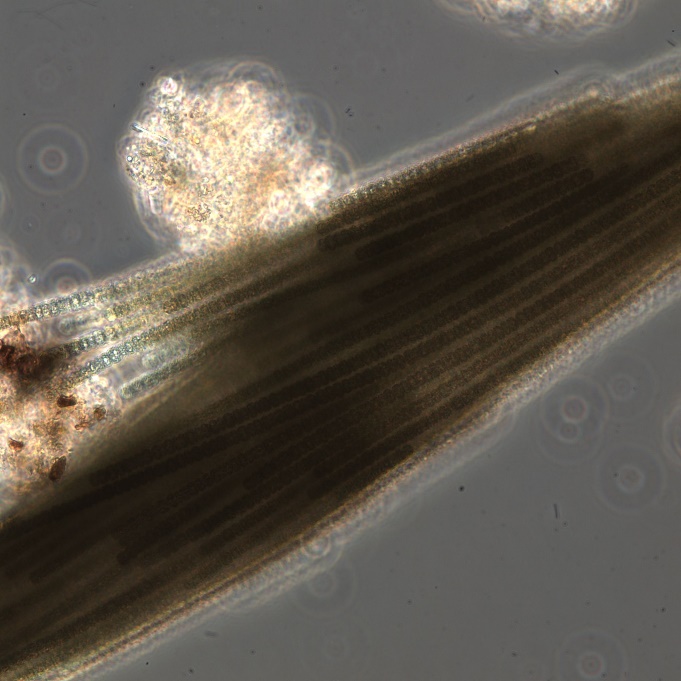
Figure A-104. Trichodesmium lacustre.
Source: Ann St Amand.
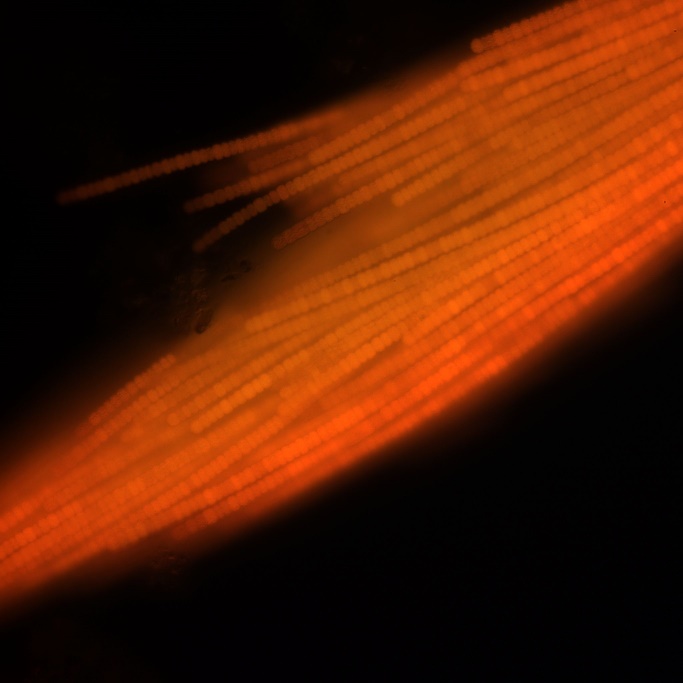
Figure A-105. Trichodesmium lacustre, epifluorescence, blue light excitation.
Source: Ann St. Amand.
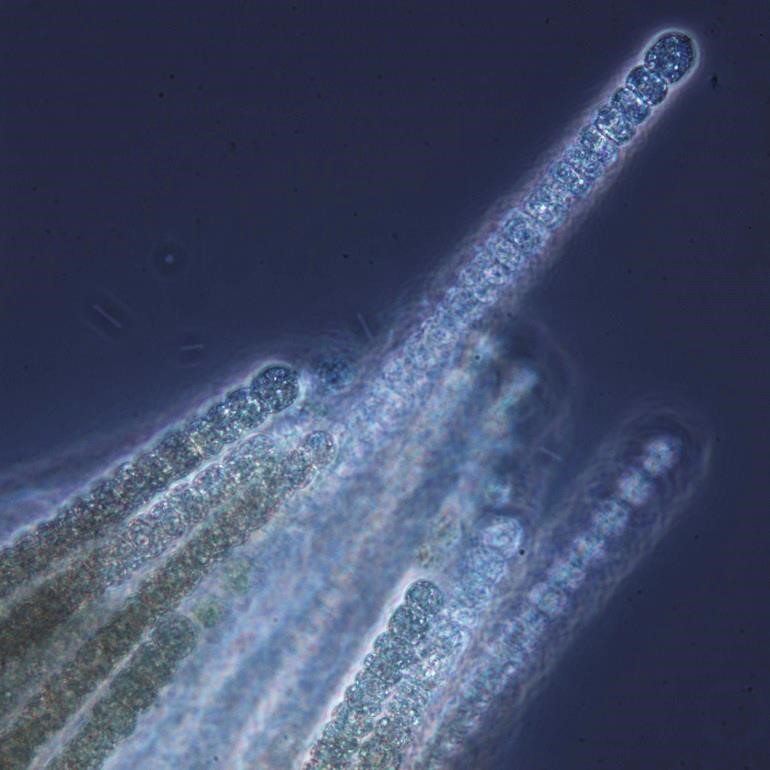
Figure A-106. Trichodesmium lacustre.
Source: Ann St. Amand.
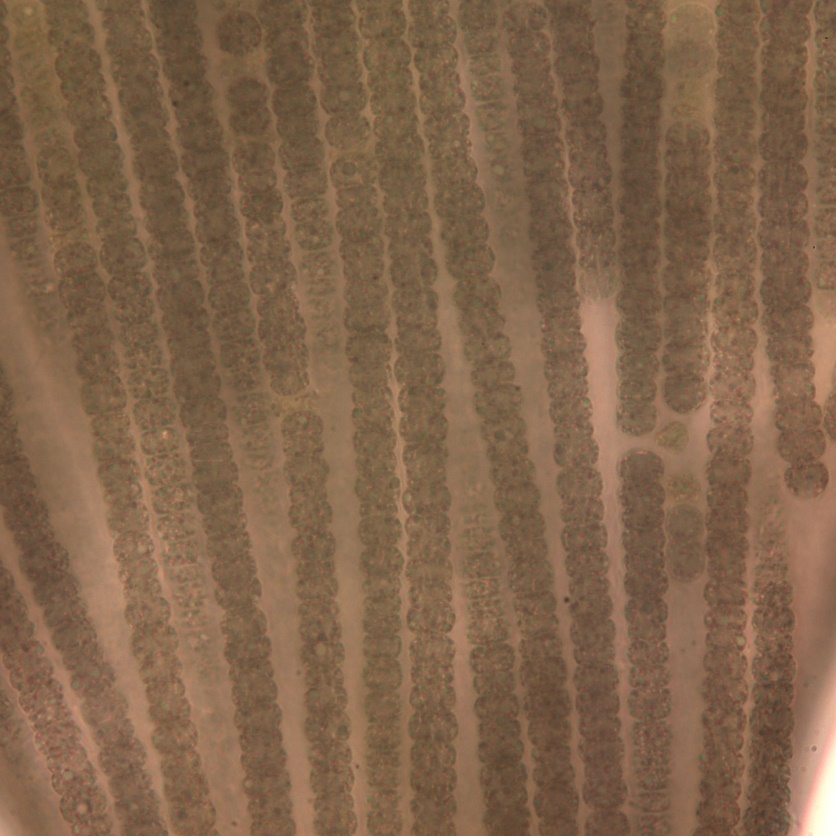
Figure A-107. Trichodesmium lacustre.
Source: Ann St. Amand.
A-7 Benthic Cyanobacteria – Field and Microscopic Images
Benthic growths are more difficult to characterize than planktonic blooms for many reasons. First, the presence and dominance of benthic growths are often difficult to see from the surface or shore. Second, benthic growths, both in streams and lakes, are much more patchy than planktonic blooms. This is because of the highly variable structures in benthic habitats, including the presence of macrophytes, boulders, trash, and other underwater objects, as well as the variability in flow regime due to grade and stream order. In addition to the physical complexity of the environment, benthic or periphytic growths are generally diverse within the benthic mat, and the taxonomy itself is still changing and complicated. Because of these issues, it is difficult to find, quantify, and identify benthic cyanotoxin producers. As with planktonic taxa, taste and odor compounds are often produced by benthic taxa, but taste and odor production does not mean that the same taxa are also producing cyanotoxins.

Figure A-108. Microcoleus/Tychonema, Clackamas River at McIver Park, OR.
Photo: Kurt Carpenter.
Anabaena sp. – Bloom images

Figure A-109. Anabaena sp., Eel River watershed, CA.
Photo: Keith Bouma-Gregson.

Figure A-110. Anabaena sp., Eel River watershed, CA.
Photo: Keith Bouma-Gregson.

Figure A-111. Anabaena sp., Eel River watershed, CA.
Photo: Keith Bouma-Gregson.
Anabaena – Description and Microscopic Images
Description: Trichomes can be single, with or without a sheath, or multiple within a sheath. Cells are often 6–8 µm wide, and 6–8 µm long. End cells can be rounded or slightly attenuated. Constricted at the cross walls, which can be slightly granulated. Without aerotopes. Trichomes are not motile. Morphological identification is the tool most often used in identification.
Secondary Compounds: anatoxin, microcystin, taste and odor compounds
Growth Habit: Forms periphytic growths that are often bright or dark blue-green. Mats will occasionally float to the surface. These periphytic mats are often taxonomically complex, with multiple genera and species present.

Figure A-112. Anabaena sp., Eel River watershed, CA.
Photo: Keith Bouma-Gregson.
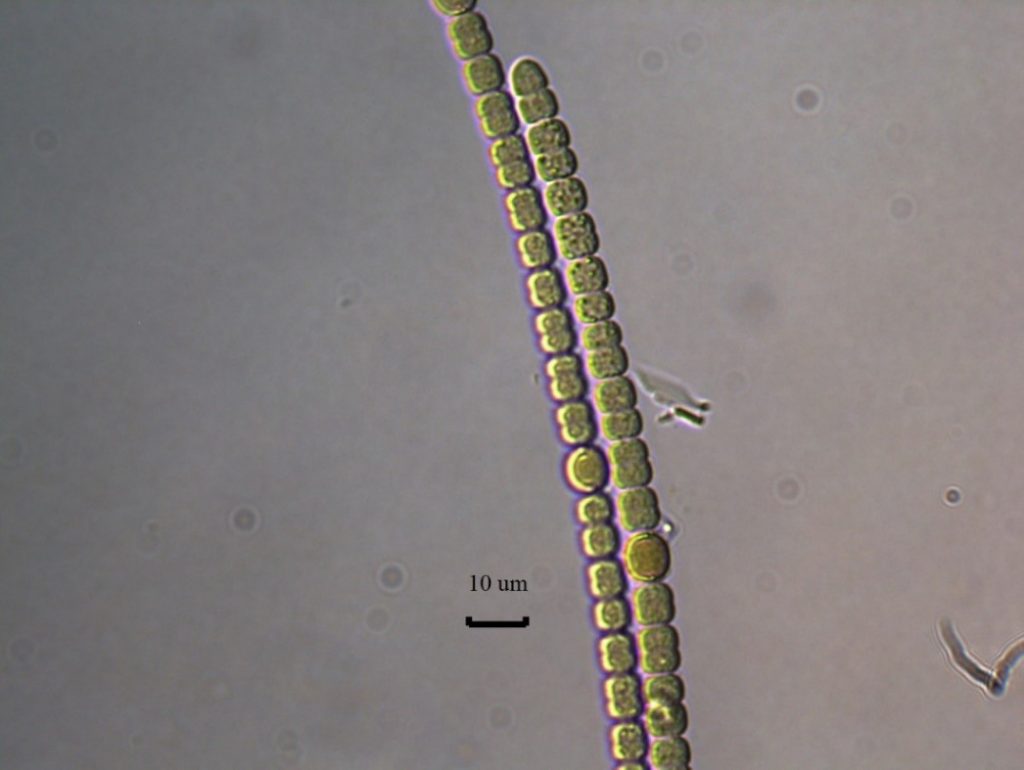
Figure A-113. Anabaena sp., Eel River watershed, CA.
Photo: Keith Bouma-Gregson.

Figure A-114. Anabaena sp., Eel River watershed, CA.
Photo: Keith Bouma-Gregson.
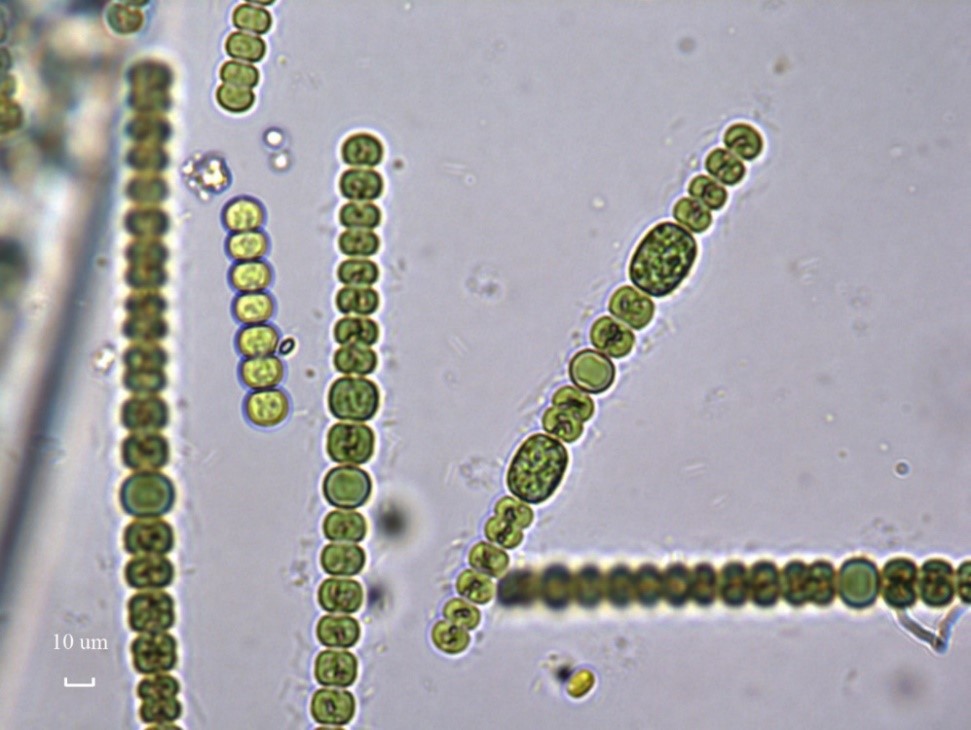
Figure A-115. Anabaena sp., Eel River watershed, CA.
Photo: Keith Bouma-Gregson.
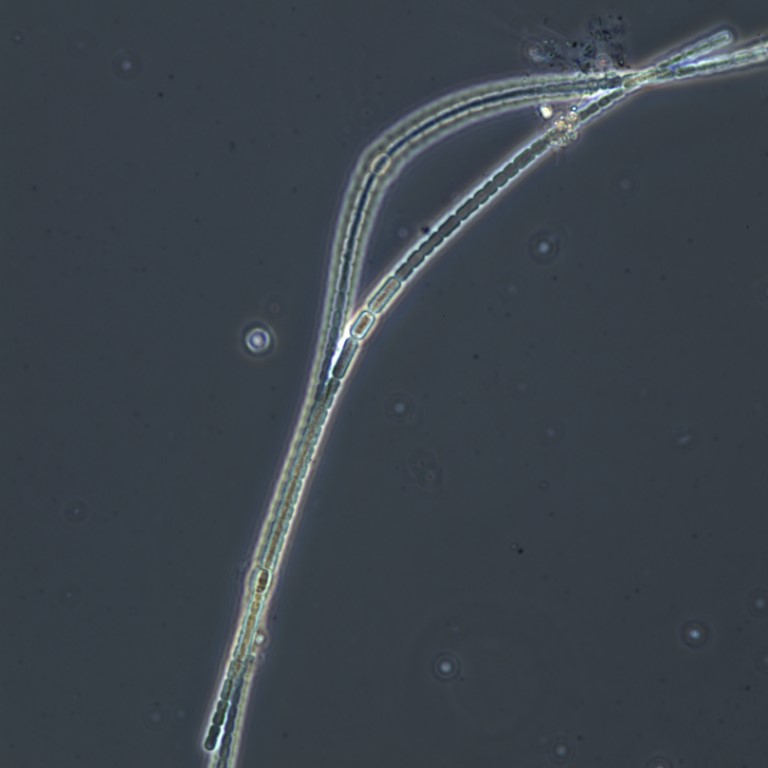
Figure A-116. Anabaena sp., Zion National Park, UT.
Photo: Ann St. Amand.
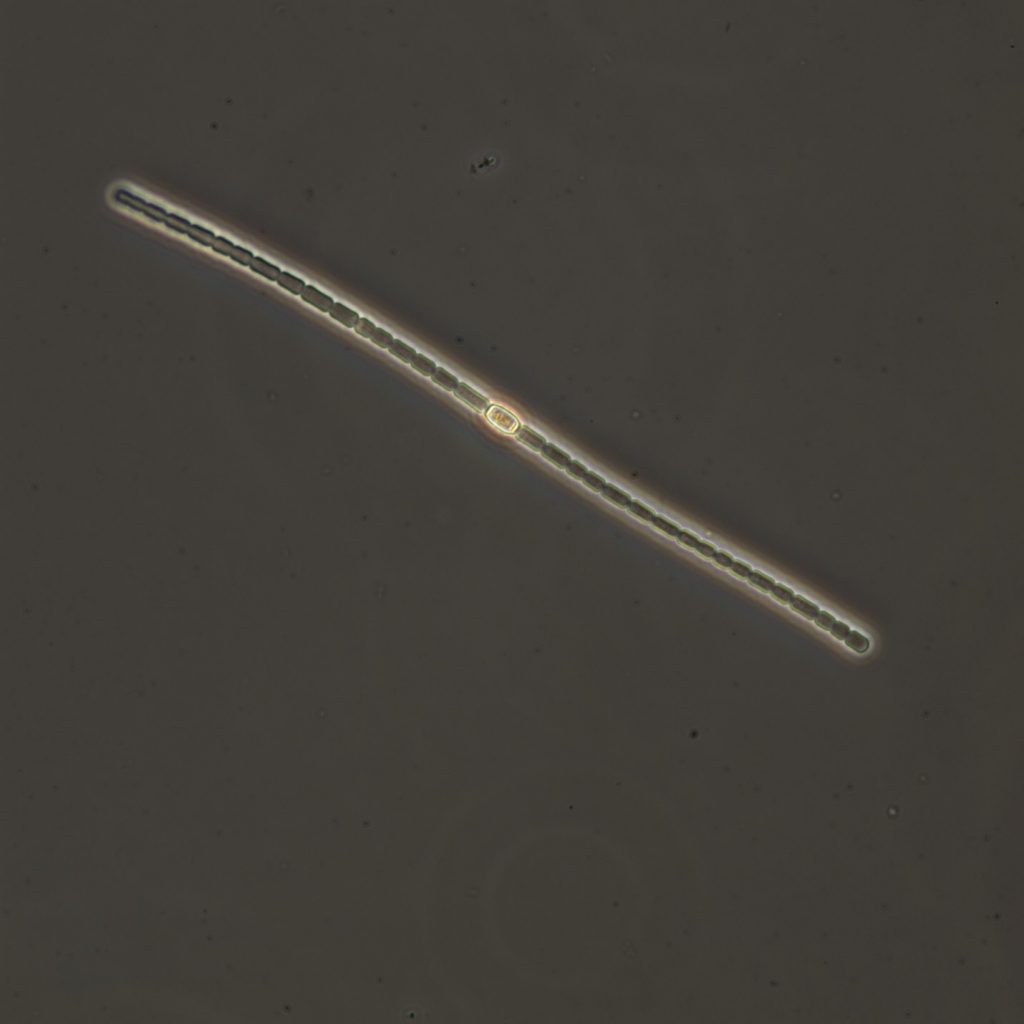
Figure A-117. Anabaena sp., Zion National Park, UT.
Photo: Ann St. Amand.
Microseira (Lyngbya/Plectonema) wollei – Bloom images

Figure A-118. Microseira wollei (left), Spirogyra sp. (filamentous green algae, right).
Photo: Gina LaLiberte.

Figure A-119. Microseira wollei, Lake Waubesa, WI.
Photo: Gina LaLiberte.

Figure A-120. Microseira wollei, Lake Erie. OH. Trichomes may be rolled into balls by wave action.
Photo: Gina LaLiberte.
Microseira (Lyngbya/Plectonema) wollei – Description and Microscopic Images
Description: Greenish-black woolly mat of densely aggregated filaments, much like steel wool. Filaments straight to variously contorted, rarely false branched. Sheath clearly lamellated (roughened) and often colored with age, open at the apex. Trichomes isopolar, uniformly cylindrical, up to 70 µm wide; often encrusted with epiphytes. Not or slightly constricted at the cross walls. Cells discoid, 5–15 times broader than long; contents finely granular, without aerotopes; apical cells rounded.
Secondary Compounds: lyngbyatoxin, saxitoxin
Growth Habit: Forms periphytic growths that often look like diatom scums. Can look like dark blue-green, red-brown, yellow-brown, or brown films on substrates or macrophytes, and often looks “ridged.” Mats will occasionally float to the surface. These periphytic mats are often taxonomically complex, with multiple genera and species present.
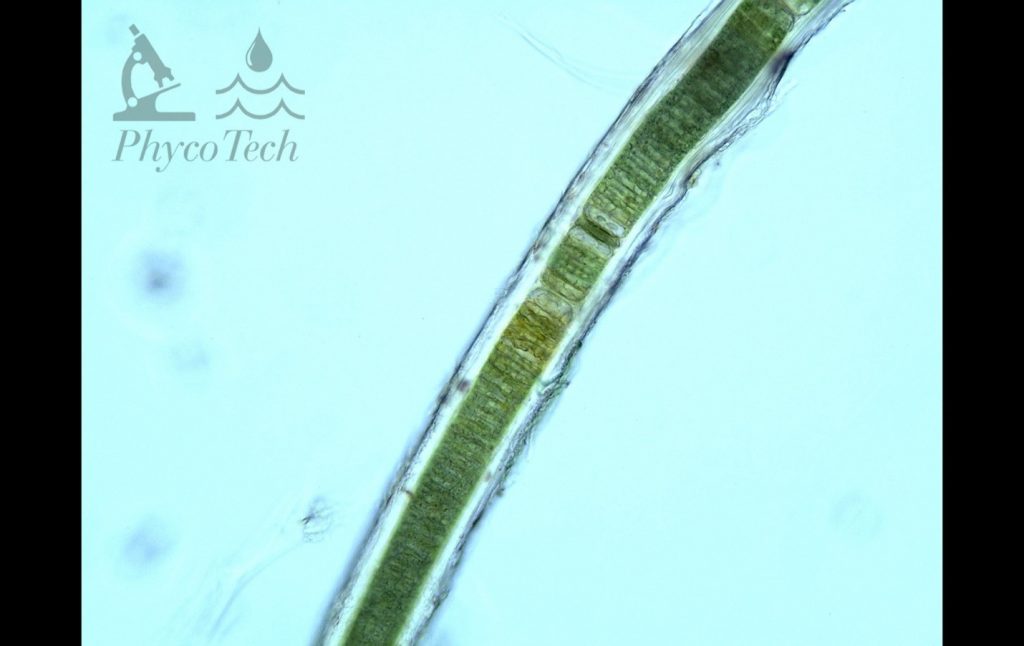
Figure A-121. Microseira wollei, laminated sheath.
Photo: Ann St. Amand.

Figure A-122. Microseira wollei, Lake Erie. False branching.
Photo: Gina LaLiberte.

Figure A-123. Microseira wollei, laminated sheath.
Photo: Ann St. Amand.
Microcoleus/Tychonema– Benthic Bloom Images

Figure A-124. Microcoleus/Tychonema, Zion National Park, UT. Note how these taxa form a thin layer that spreads over sediment and aquatic plants.
Photo: Robyn Henderek

Figure A-125. Microcoleus/Tychonema, Zion National Park, UT. Note the layer located at the water’s edge.
Photo: Robyn Henderek.



Figure A-126. Microcoleus/Tychonema, Zion National Park, UT.
Photo: Robyn Henderek.

Figure A-127. Microcoleus/Tychonema, Zion National Park, UT.
Photo: Robyn Henderek.

Figure A-128. Microcoleus/Tychonema, Zion National Park, UT.
Photo: Robyn Henderek.

Figure A-129. Microcoleus/Tychonema, Zion National Park, UT. The bulbous appearance of the mat can occur when oxygen released during photosynthesis is trapped among the trichomes. Other benthic species may look similar; see A-184.
Photo: Robyn Henderek.
Microcoleus/Tychonema – Description and Microscopic Images
Description: Trichomes can be single, with or without a sheath, or multiple within a sheath. Cells are often 6–8 µm wide and 6–8 µm long. End cells can be rounded or slightly attenuated. Not or only slightly constricted at the cross walls, which can be slightly granulated. Without aerotopes. Tychonema can have widened thylakoids that appear like small “vacuoles,” and often have slightly “keritomized” cell contents as well. Trichomes are often motile. These genera are difficult to separate and often co-occur. Genetic data has thus far been sparse, so morphological identification is the tool most often used in identification.
Secondary Compounds: anatoxin, microcystin, taste and odor compounds
Growth Habit: Forms periphytic growths that often look like diatom scums. Can look like dark blue-green, red-brown, yellow-brown, or brown films on substrates or macrophytes, and often looks “ridged.” Mats will occasionally float to the surface. These periphytic mats are often taxonomically complex, with multiple genera and species present.
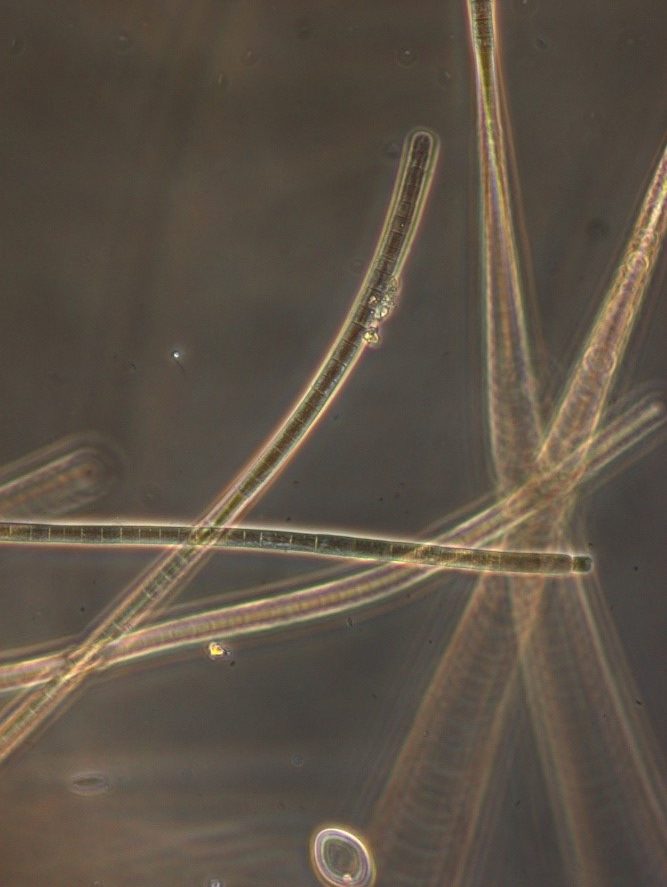
Figure A-130. Microcoleus/Tychonema, Zion National Park, UT.
Photo: Ann St. Amand.
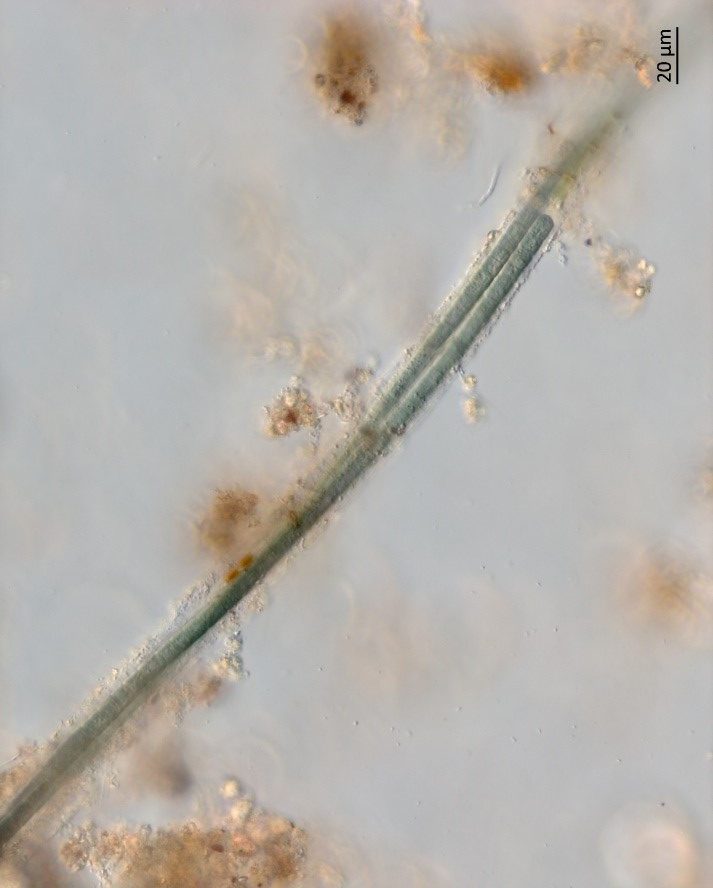
Figure A-131. Microcoleus/Tychonema, Zion National Park, UT.
Photo: Barry Rosen.

Figure A-132. Microcoleus/Tychonema, Zion National Park, UT.
Photo: Rosalina Hristova.

Figure A-133. Microcoleus/Tychonema, Zion National Park, UT.
Photo: Barry Rosen.
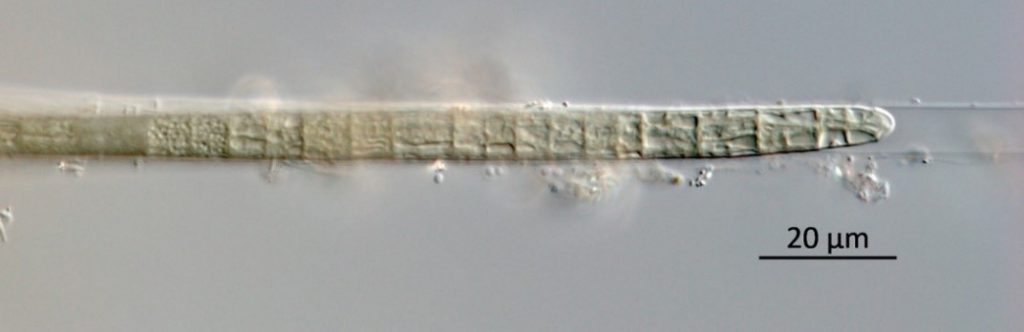
Figure A-134. Microcoleus/Tychonema, Zion National Park, UT.
Photo: Barry Rosen.
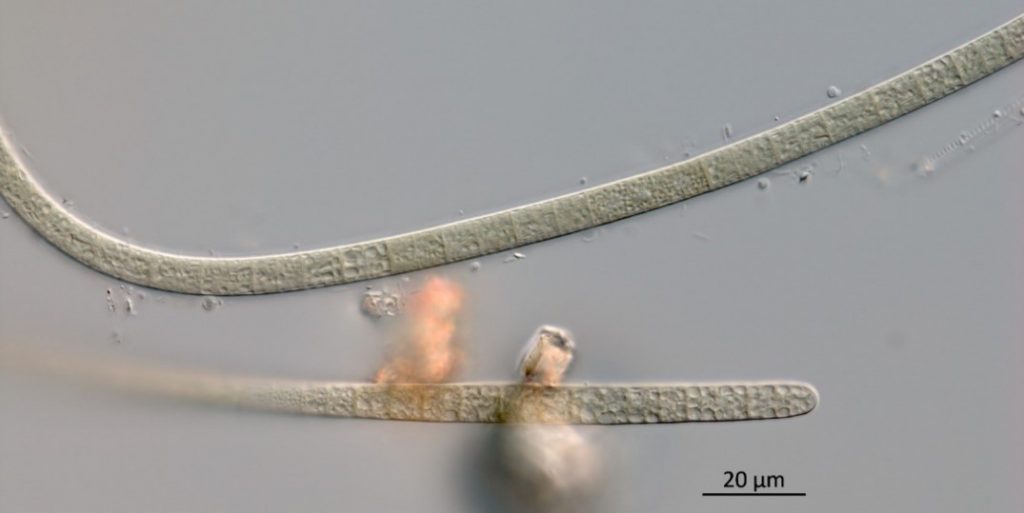
Figure A-135. Microcoleus/Tychonema, Zion National Park, UT.
Photo: Barry Rosen.
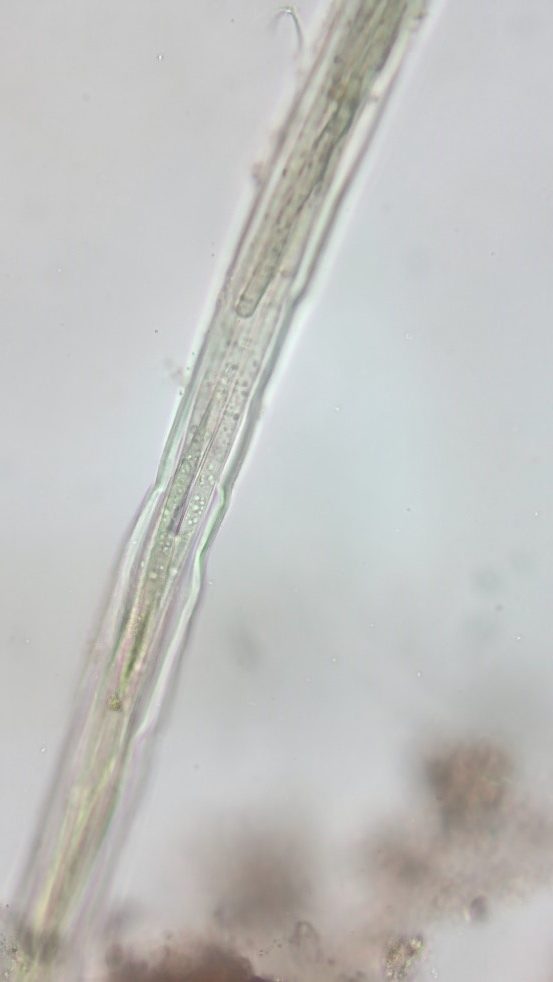
Figure A-136. Microcoleus/Tychonema, Zion National Park, UT.
Photo: Kurt Carpenter.

Figure A-137. Microcoleus/Tychonema, Zion National Park, UT.
Photo: Kurt Carpenter.
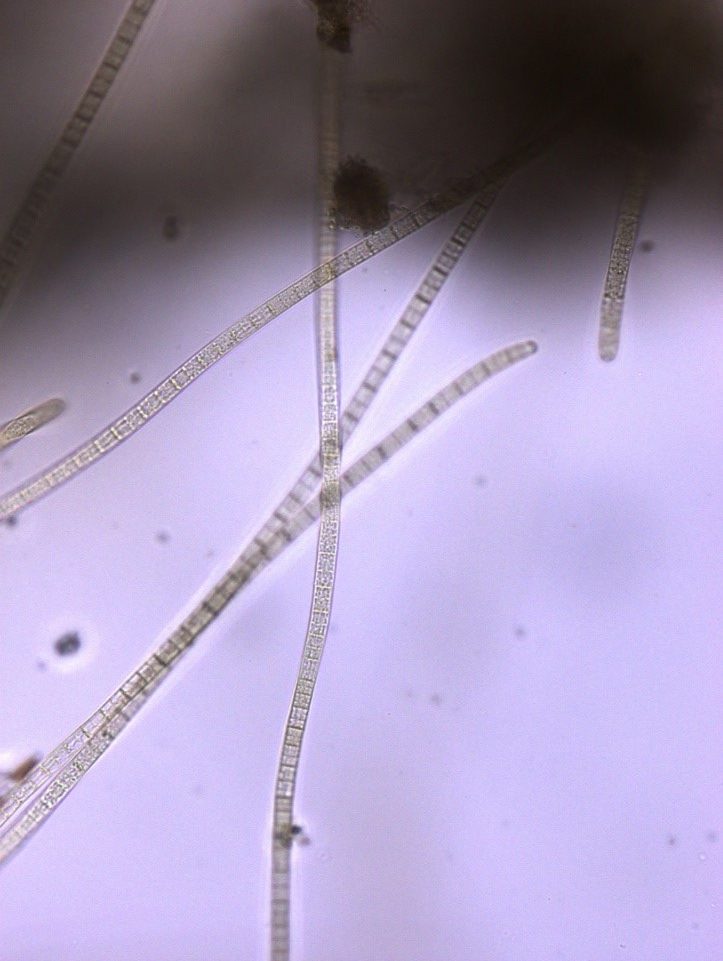
Figure A-138. Microcoleus/Tychonema sp., Zion National Park, UT.
Photo: Ann St. Amand.
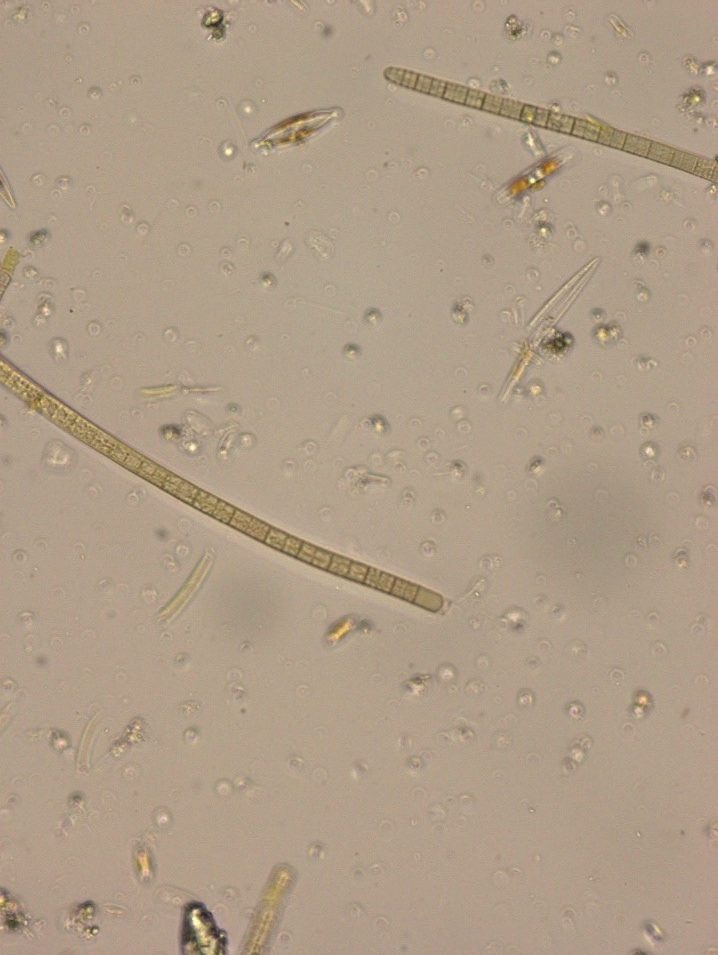
Figure A-139. Microcoleus/Tychonema sp., Zion National Park, UT.
Photo: Ann St. Amand.
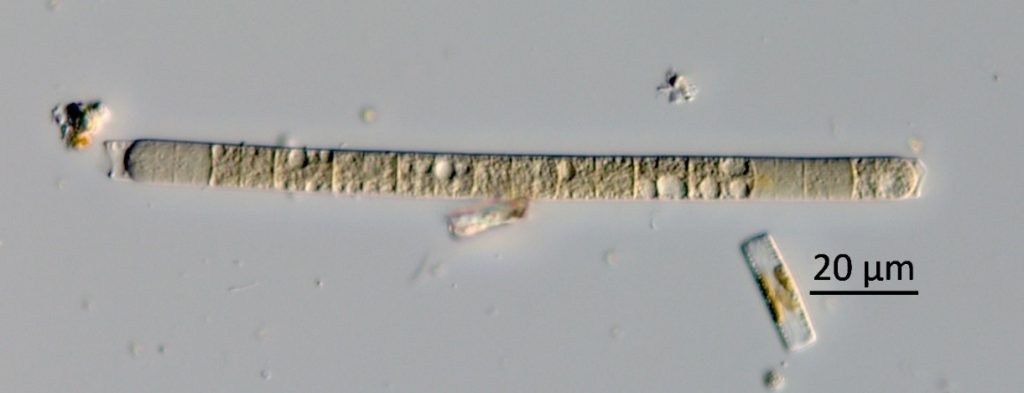
Figure A-140. Microcoleus/Tychonema sp., Zion National Park, UT.
Photo: Barry Rosen.

Figure A-141. Microcoleus autumnale, Zion National Park, UT.
Photo: Ann St. Amand.

Figure A-142. Microcoleus autumnale, Zion National Park, UT.
Photo: Ann St. Amand.
Oscillatoria sp. – Benthic Bloom Images

Figure A-143. Oscillatoria sp., Stony Lake, MI. Note the air bubbles trapped in this dark green mat.
Photo: Ann St. Amand.

Figure A-144. Oscillatoria sp., OR.
Photo: Kurt Carpenter.

Figure A-145. Oscillatoria sp., WA
Photo: Kurt Carpenter.

Figure A-146. Oscillatoria sp., OR.
Photo: Kurt Carpenter.
Oscillatoria – Description and Microscopic Images
Description: Filaments solitary or more commonly aggregated into mats, which often look “felt- or leather-like.” Mats can range from light to dark blue-green to red or purple. Trichomes may or may not have individual sheaths, which are facultative and only produced under stress. Trichomes are often motile with gliding, oscillation, or left/right-handed rotation. Cells are always significantly shorter than wide, from 2x up to 11x. Cells are at least 6.8 µm, up to 70 µm, wide, and rarely constricted. Cell walls or contents can be granulated. Without aerotopes. The end of the trichome may be bent/curved and end cells may be attenuated.
Secondary Compounds: cylindrospermopsin, microcystin, anatoxin-a, aplysiatoxin, lipopolysaccharide
Growth Habit: Forms mats attached to hard substrates or macrophytes in streams or rivers or in softer sediments among macrophytes, mosses, and other algae in littoral areas. Mats often become detached later in the season and float to the surface, can be mixed with Phormidium.
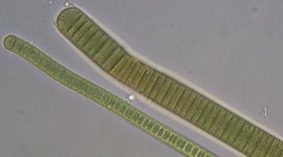
Figure A-147. Oscillatoria sp., OR.
Photo: Kurt Carpenter.
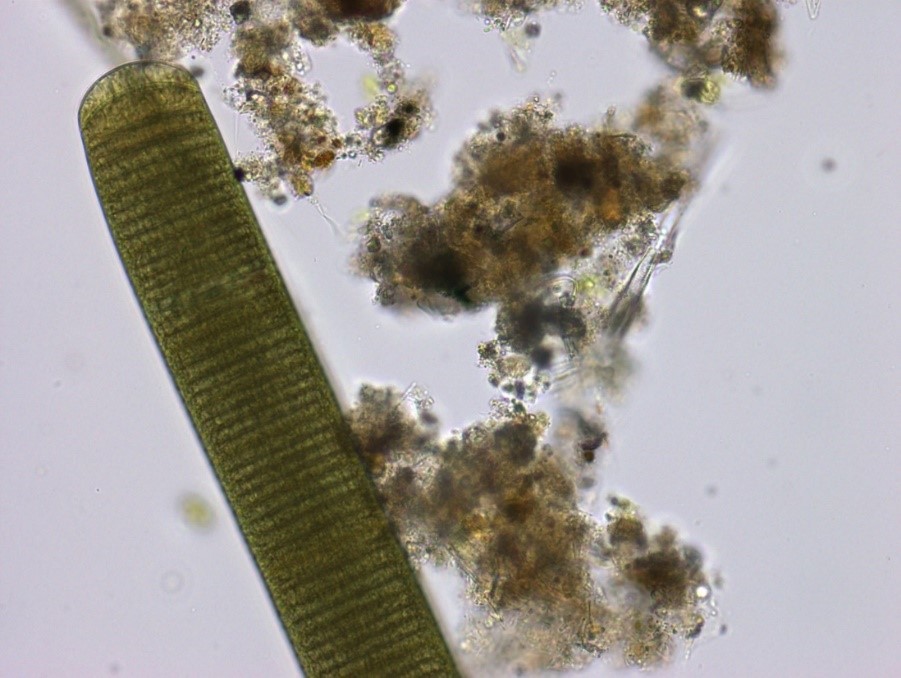
Figure A-148. Oscillatoria sp. with organic debris. Note the “stacked coin” appearance of the trichome.
Photo: Ann St. Amand.
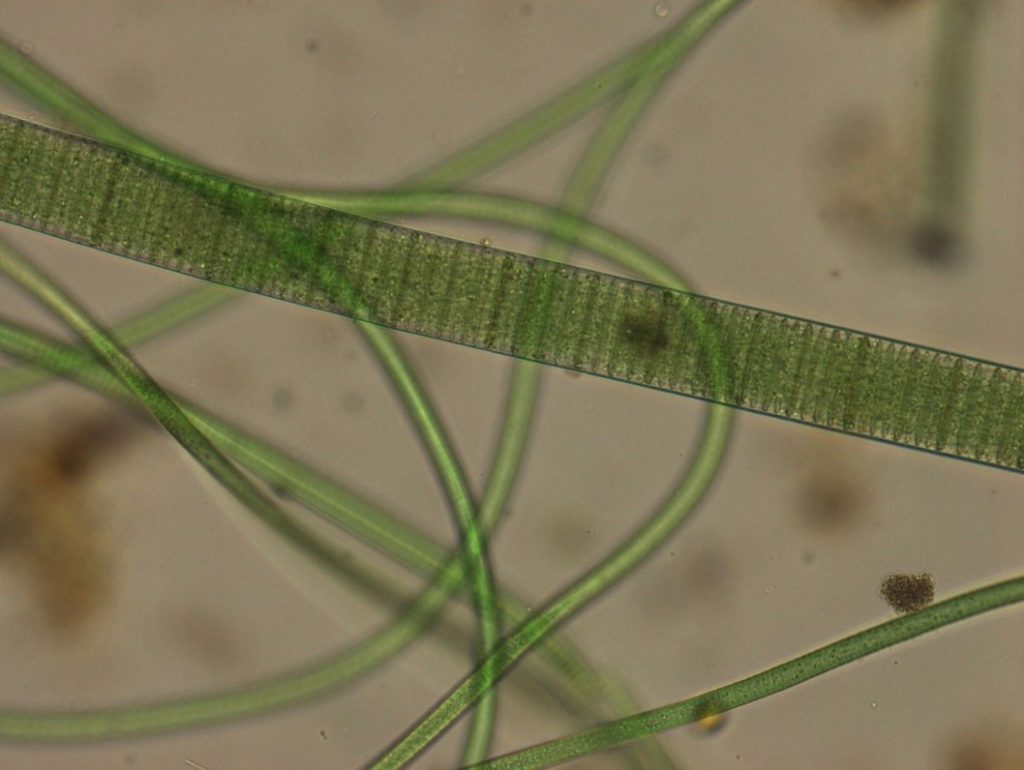
Figure A-149. The large filament in this photo is Oscillatoria sp. Note the stacked coin-like appearance within the filament.
Photo: Ann St. Amand.
Phormidium sp. – Bloom images

Figure A-150. Phormidium sp., Zion National Park, UT.
Photo: Hannah Bonner.

Figure A-151. Phormidium sp.
Photo: Keith Bouma-Gregson.
Phormidium sp. – Description and Microscopic Images
Description: Filaments solitary or more commonly aggregated into mats, which often look “felt- or leather-like.” Mats can range from light to dark blue-green to red or purple. Trichomes may or may not have individual sheaths, which are facultative and only produced under stress. Never branching. Trichomes are often motile with gliding, oscillation, or left/right-handed rotation. Cells are more or less isodiametric. Cells 2–14 µm, and rarely constricted. Cell walls or contents can be granulated. Without aerotopes. The end of the trichome may be bent/curved and end cells may be attenuated.
Secondary Compounds: microcystin, saxitoxin
Growth Habit: Forms mats attached to hard substrates or macrophytes in streams or rivers or in softer sediments among macrophytes, mosses, and other algae in littoral areas. Mats often become detached later in the season and float to the surface, can be mixed with Oscillatoria and Microcoleus.
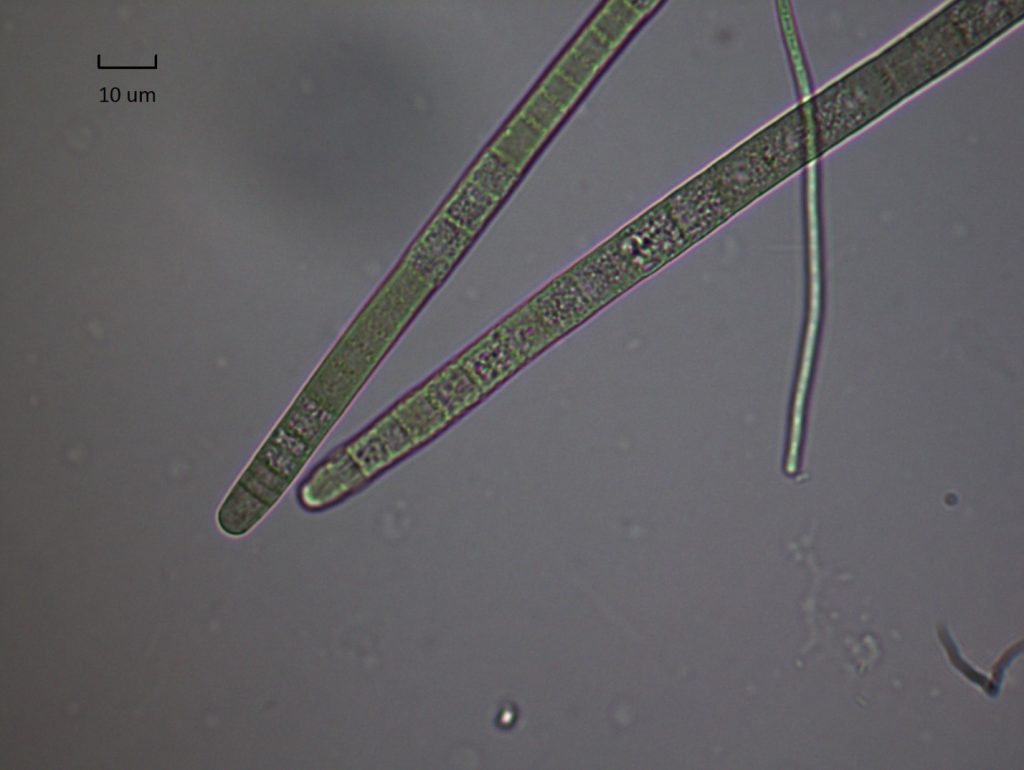
Figure A-152. Phormidium sp.
Photo: Keith Bouma-Gregson.
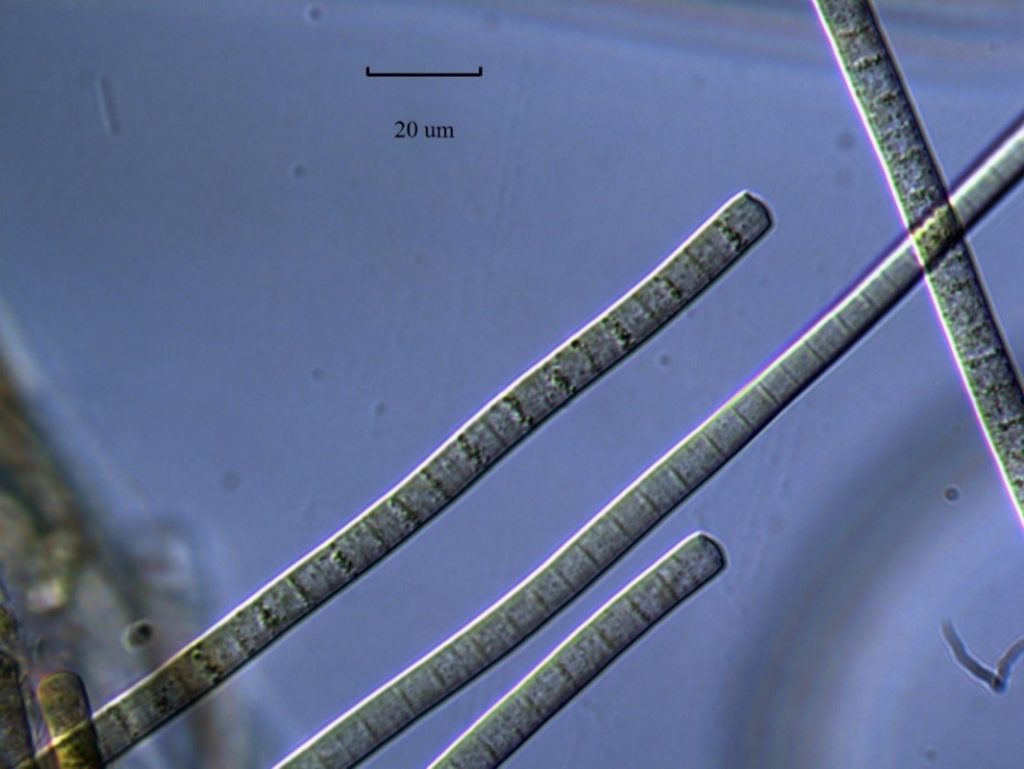
Figure A-153. Phormidium sp.
Photo: Keith Bouma-Gregson.
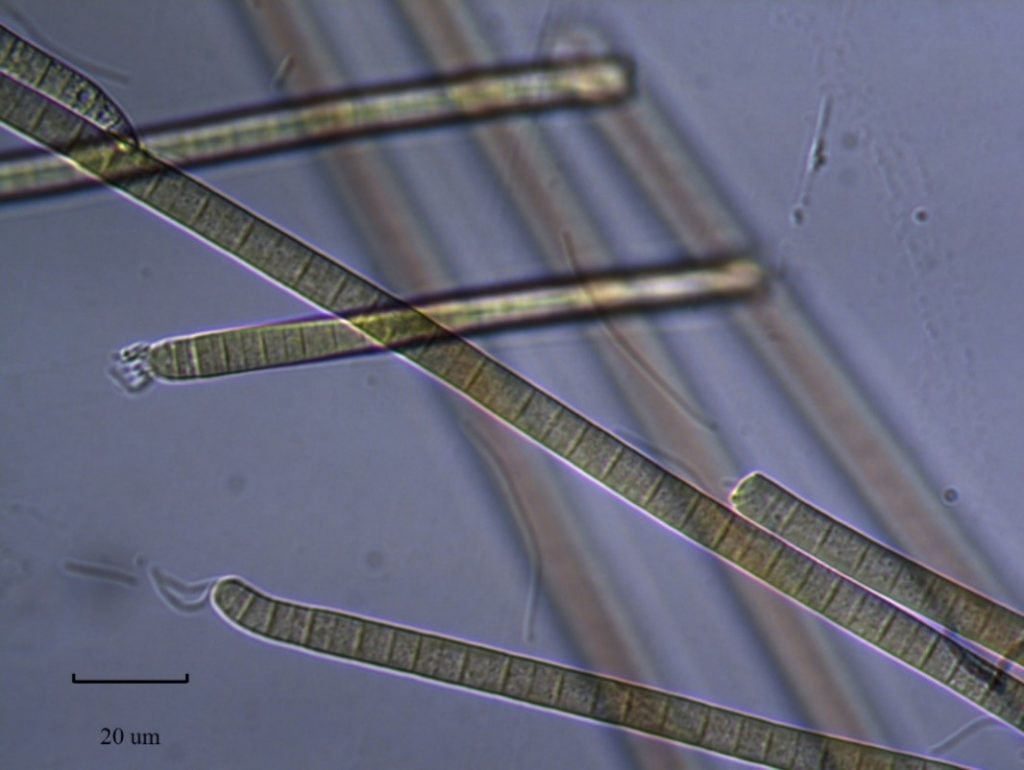
Figure A-154. Phormidium sp.
Photo: Keith Bouma-Gregson.
Nostoc sp. – Bloom images

Figure A-155. Nostoc sp., OR.
Photo: Kurt Carpenter.

Figure A-156. Nostoc sp., OR.
Photo: Kurt Carpenter.

Figure A-157. Nostoc sp. (dark nodules) and filamentous green algae (bright green filaments), OR.
Photo: Kurt Carpenter.

Figure A-158. Nostoc sp., Zion National Park, UT.
Photo: Kam Truhn.

Figure A-159. Nostoc sp. (dark nodules), Zion National Park, UT, La Verkin Creek.
Photo: Robyn Henderek.

Figure A-160. Nostoc sp., Zion National Park, UT.
Photo: Robyn Henderek.
Nostoc sp. – Description and Microscopic Images
Description: Filaments aggregated and entangled irregularly in a gelatinous colony that can often be seen with the naked eye. Colony has a firm periderm, or outer gelatinous envelope, which can be round, lobate (ears), or amorphous. Benthic species without aerotopes. Trichomes may or may not have individual sheaths and are not motile. Cells are often spherical or barrel shaped, 3–6 µm long. Heterocytes are round, and akinetes are often rare or unknown. Identification past the genus level is difficult. Colonies range from light brown to dark blue-green.
Secondary Compounds: cylindrospermopsin, microcystin, anatoxin-a, saxitoxin, nodularin, BMAA
Growth Habit: Forms gelatinous colonies attached to hard substrates in streams or rivers, or in softer sediments among macrophytes and mosses in littoral areas. Can also be found in moist terrestrial locations.
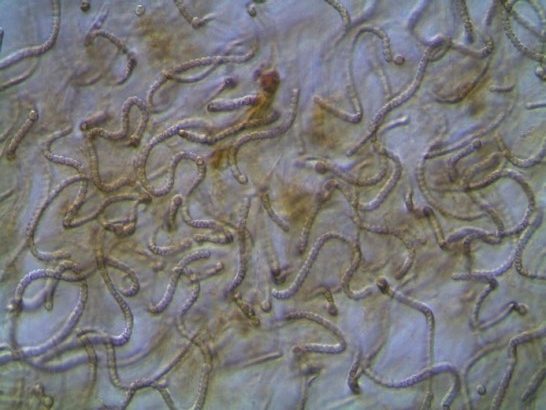
Figure A-161. Nostoc sp.
Photo: Kurt Carpenter.
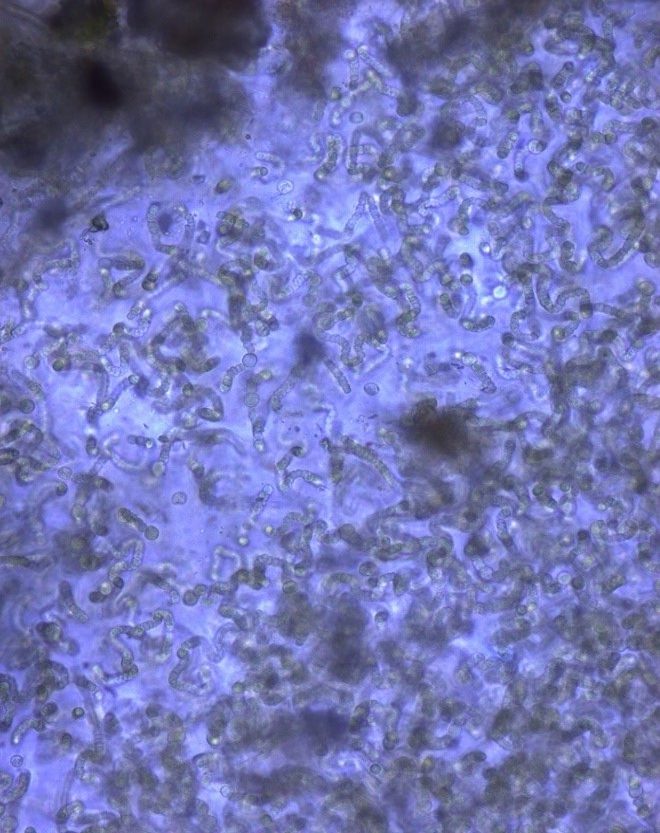
Figure A-162. Nostoc sp., Zion National Park, UT.
Photo: Ann St. Amand.
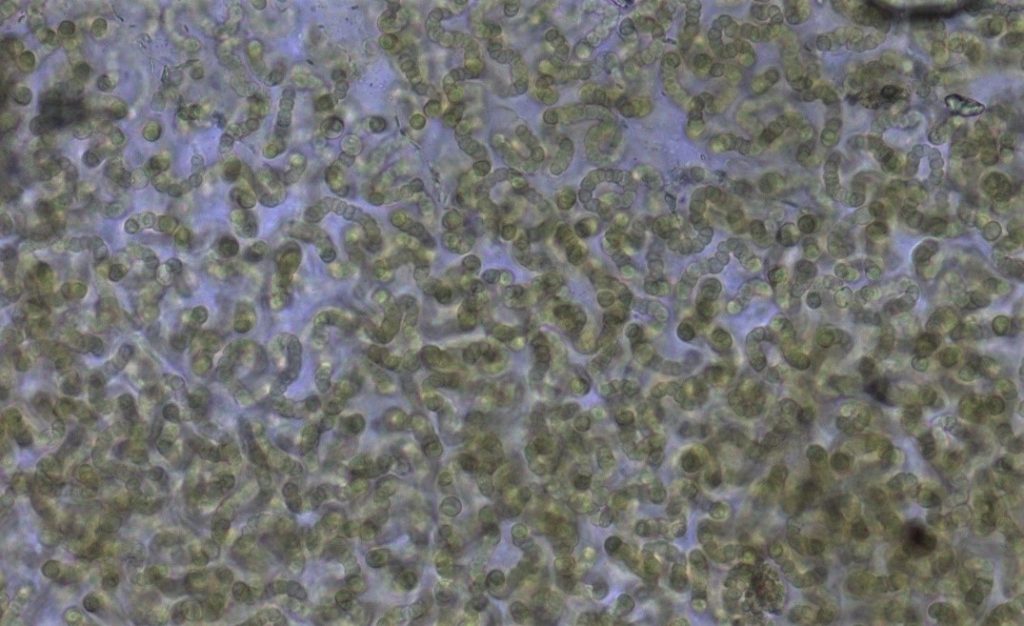
Figure A-163. Nostoc sp., Zion National Park, UT.
Photo: Ann St. Amand.
Scytonema sp.– Bloom Image

Figure A-164. Scytonema sp., arrangement of the trichomes.
Photo: Barry Rosen.
Scytonema sp. – Description and Microscopic Images
Description: Trichome about 12–30 µm wide, filament 18–30 µm wide with sheath. Cells short, 4–11 µm long. Can have slight constrictions, giving the filament a “stacked disk or coin appearance.” Heterocytes are short cylindrical or ellipsoidal. False branches, when present, do not occur at heterocytes.
Secondary Compounds: cylindrospermopsin, guanitoxin, microcystin, saxitoxin
Growth Habit: Benthic mats grow up from the bottom, break free, and accumulate along the shoreline. Note that these can be picked up with a stick. Unlike filamentous green algae or Microseira wollei, these mats have a cottony texture and break apart into short pieces. They do not pull apart into hairlike strands.
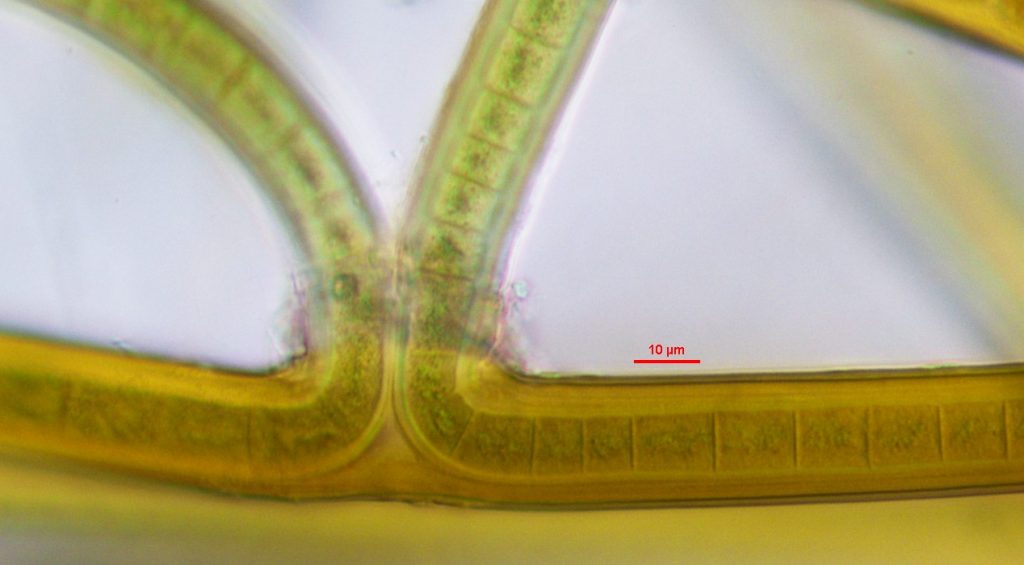
Figure A-165. Scytonema sp. Note the false branching that can occur in this genus. Great Smoky Mountains.
Photo: Andrew Chapman.
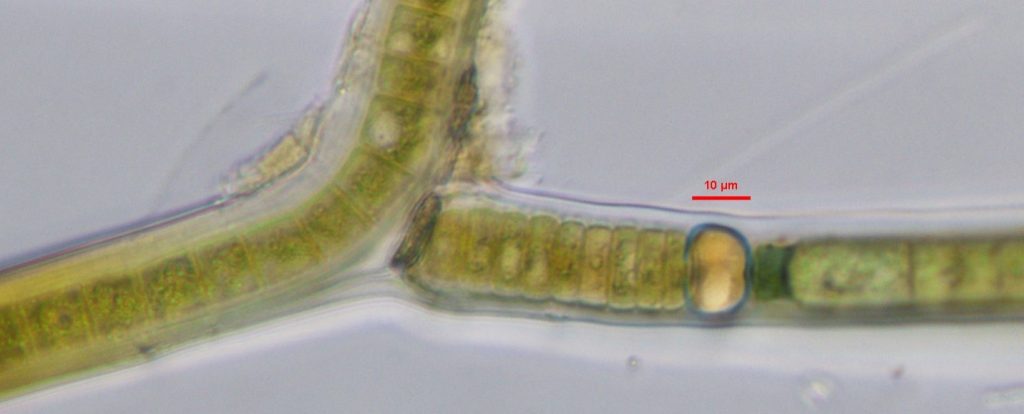
Figure A-166. Scytonema sp. Note the false branch and the heterocytes. Great Smoky Mountains.
Photo: Andrew Chapman.
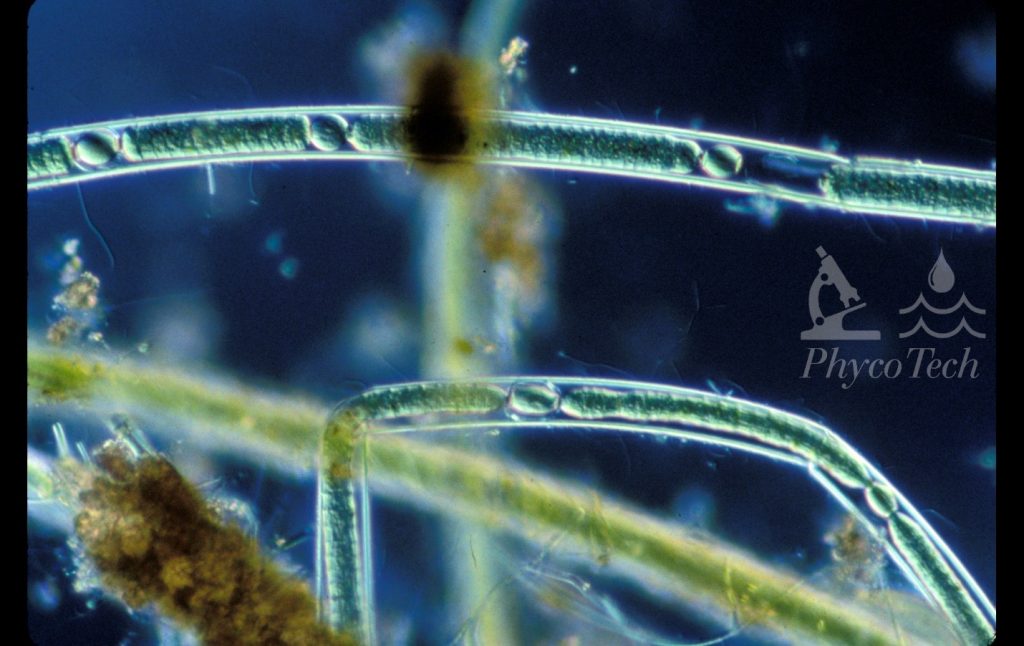
Figure A-167. Scytonema sp., darkfield. Note the visible sheath and heterocytes.
Photo: Ann St. Amand.
Wollea sp. – Microscopic Image
Description: Consists of multiple trichomes in a colonial sheath that are often tube- or sack-like. It tends to appear in diverse algal mats, like in the mats of stalked diatoms such as Cymbella janischii. Trichomes are often parallel. Cells are barrel-shaped to cylindrical without aerotopes. Akinetes are next to heterocytes.
Secondary Compounds: cylindrospermopsin, microcystin, saxitoxin
Growth Habit: Attached to benthic substrates or aquatic macrophytes, and sometimes detached and floating late in the season.

Figure A-168. Wollea sp., Clackamas River.
Photo: Barry Rosen.
A-8 Nontoxic Surface and Subsurface Accumulations: Field Images and Microscopic Images
Not all accumulations are harmful. In fact, their presence may indicate a healthy, thriving ecosystem. Many organisms other than cyanobacteria are in or near water. Algae and other aquatic plants at the base of the food chain are common in lakes, ponds, and rivers. Land and wetland plants may shed pollen or seeds. Zooplankton and aquatic insects live in the water and occasionally accumulate in eye-catching masses.
It is often difficult to distinguish an HCB from a nontoxic bloom or other aquatic plants. Frequently visiting your water body will help you become familiar with what commonly grows there and how its appearance changes over the course of the year. With experience, you will feel more comfortable recognizing the more common green algae and aquatic plants you see. For additional resources, see Kannan and Lenca (2013) and Schmitt (2005).
The following pages contain field and microscopic images of several common nontoxic algae, aquatic plants, and other plant matter, including representatives from the following groups:
- chlorophytes/green algae
- diatoms
- euglenoids
- floating ferns
- floating macrophytes
- pollens
- rooted plants
- zooplankton resting spores (epipphia)
Chlorophytes/Green Algae
Cladophora sp.

Figure A-169. Chlorophyte, Cladophora sp., Carmel, IN. Cladophora sp. often forms very dense mats with a spongy texture.
Source: Ann St. Amand.

Figure A-170. Chlorophyte, Cladophora sp., Paw Paw River, MI.
Source: Ann St. Amand.

Figure A-171. Chlorophyte,
Cladophora sp., Nomarski.
Source: Ann St. Amand.
Hydrodictyon sp. (water net)

Figure A-172. Chlorophyte, Hydrodictyon sp., water net. Note: We do not recommend whole-body immersion in unknown scums!
Source: Ken Wagner.

Figure A-173. Chlorophyte, Hydrodictyon sp., water net.
Source: Ann St. Amand.
Mougeotia sp.

Figure A-174. Chlorophyte, Mougeotia sp.
Source: Steve Heiskary, Minnesota Pollution Control Agency.

Figure A-175. Chlorophyte, Mougeotia sp. This taxon has a characteristic slimy feel and hair-like texture.
Source: Steve Heiskary, Minnesota Pollution Control Agency.
Pithora sp.

Figure A-176. Chlorophyta, Pithora sp.
Source: Ann St. Amand.
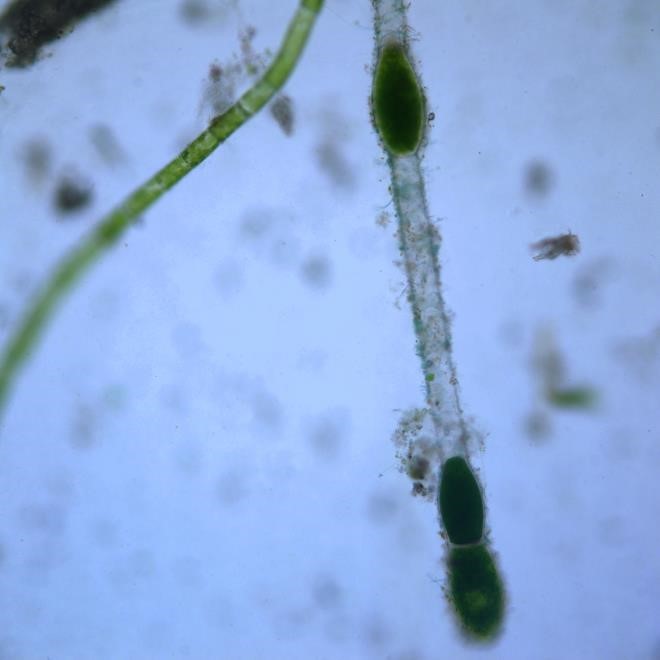
Figure A-177. Chlorophyta, Pithora sp., Nomarski.
Source: Ann St. Amand.
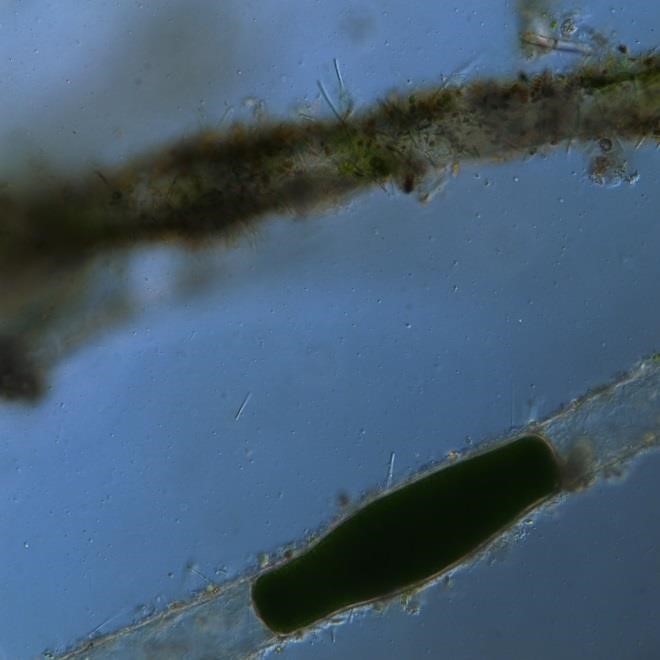
Figure A-178. Chlorophyta, Pithora sp., Nomarski.
Source: Ann St. Amand.
Spirogyra sp.

Figure A-179. Chlorophyta, Spirogyra sp. Note the fluorescent green color. This taxon has a slimy feel and hair-like texture.
Source: Ken Wagner.

Figure A-180. Chlorophyta, Spirogyra sp., bloom.
Source: Terri Peters.

Figure A-181. Chlorophyta, Spirogyra sp. Filaments showing the helical chloroplast.
Source: Ann St Amand.
Diatoms (class Bacillariophyceae)– Bloom images

Figure A-182.-Zion National Park, UT, Diatoms (Bacillariophyceae).
Photo: Hannah Bonner.

Figure A-183. Zion National Park, UT, Diatoms (Bacillariophyceae).
Photo: Hannah Bonner.

Figure A-184. Zion National Park, UT, Diatoms. (Bacillariophyceae). Other benthic species may look similar, see A-129.
Photo: Hannah Bonner
Diatoms: Description and Microscopic Images
Description: “Diatom” is a general term used for all species in the class Bacillariophyceae. Single cells or colonies in filaments, tubes, or on stalks. Colonies range from light brown to dark brown. Cells have silica dioxide incorporated into cell wall.
Secondary Compounds: geosmin, MIB
Growth Habit: forms gelatinous or billowy colonies, or easily fragmented brown filaments flowing downstream, attached to hard substrates in streams or rivers. Can grow in a dark brown, very slick surface bloom on hard substrates or in softer sediments among macrophytes and mosses in littoral areas, can also be found in moist terrestrial locations.
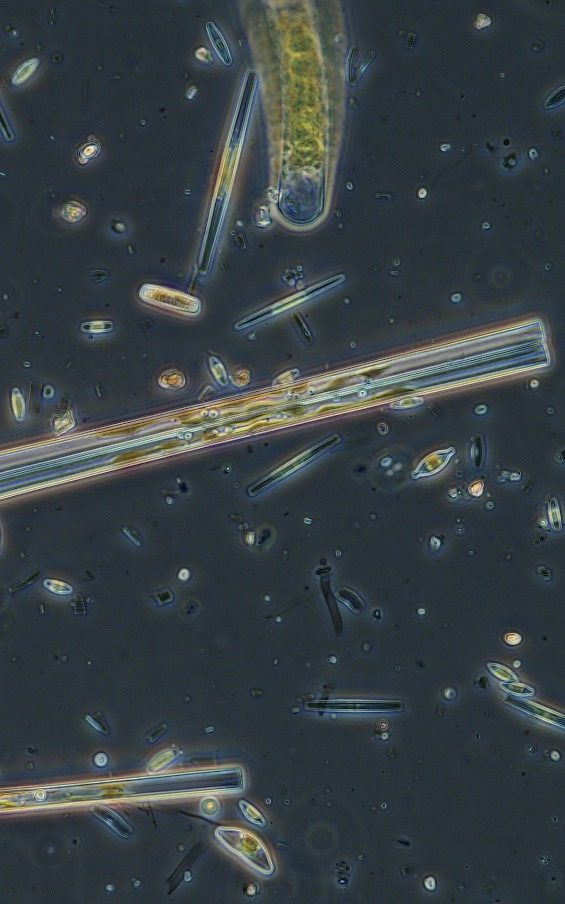
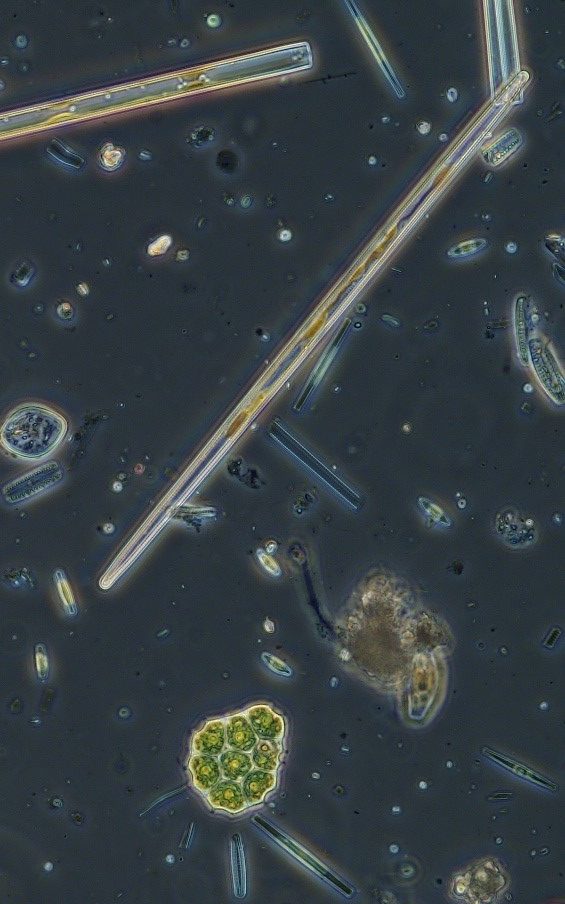
Figure A-185. Zion National Park, UT, Diatoms (Bacillariophyceae).
Photo: Ann St. Amand.
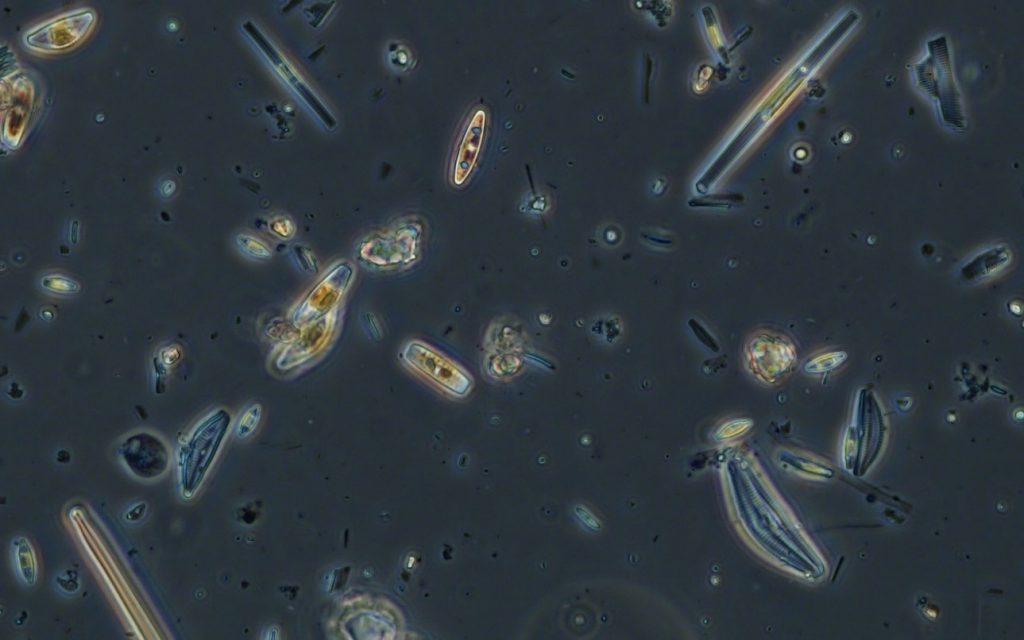
Figure A-186. Zion National Park, UT, Diatoms (Bacillariophyceae).
Photo: Ann St. Amand.
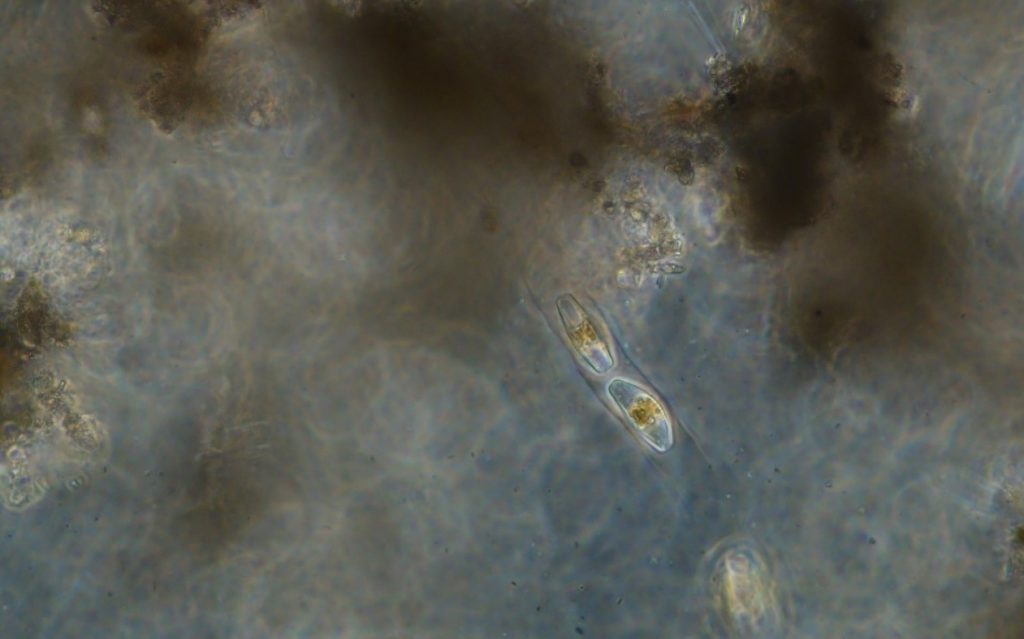
Figure A-187. Zion National Park, UT, Diatoms (Bacillariophyceae).
Photo: Ann St. Amand.
Euglenoids

Figure A-188. Euglenophyta, Euglena sp., Berrien County, MI. This taxon often forms surface blooms.
Source: Ann St. Amand.
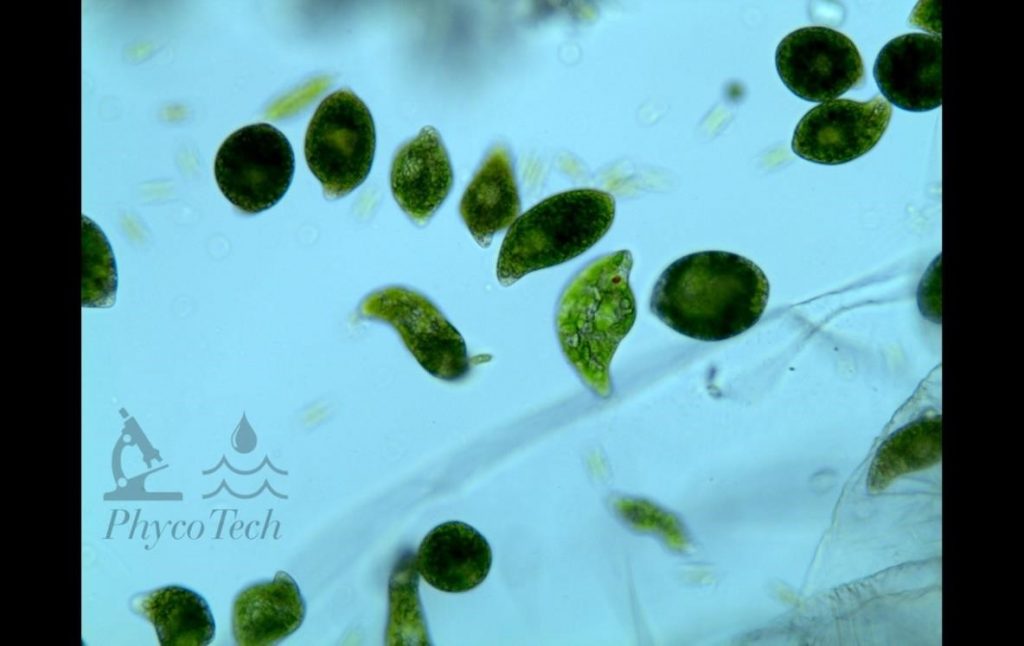
Figure A-189. Euglenophyta, Euglena sp., Berrien County.
Source: Ann St. Amand.
Floating Ferns
Azolla sp. (water fern)

Figure A-190. Azolla sp., water fern, Chicago Botanic Garden, IL.
Source: Bob Kirschner.

Figure A-191. Azolla sp., water fern with symbiotic Trichormus (Anabaena) azollae.
Source: Ann St. Amand.
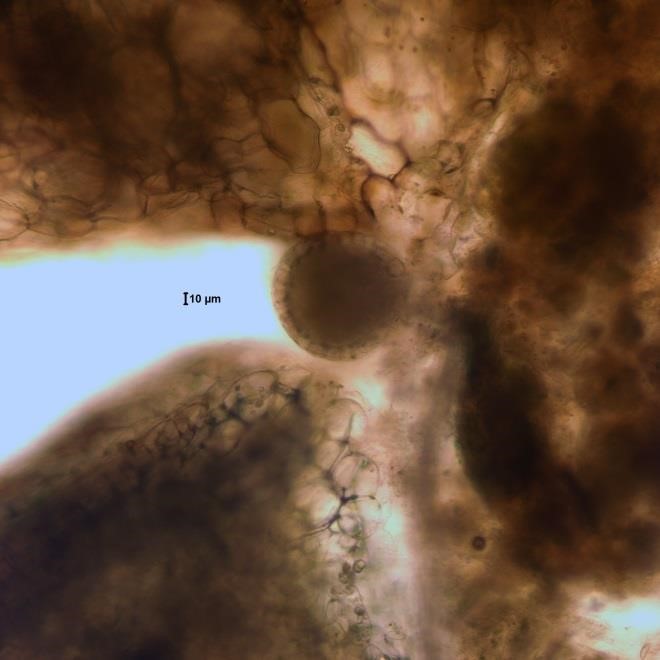
Figure A-192. Azolla sp., water fern, leaves.
Source: Ann St. Amand.
Floating Macrophytes
Lemna sp. (duckweed)

Figure A-193. Lemna sp., Kalamazoo County, MI. Duckweed often forms surface blooms of tiny, but visible, floating leaves.
Source: Ann St. Amand.

Figure A-194. Lemna sp., Kalamazoo County, MI. Duckweed showing separated plants.
Source: Ann St. Amand.
Wolffia sp. (watermeal)

Figure A-195. Wolffia columbiana, watermeal, Kalamazoo County, MI.
Source: Ann St. Amand.
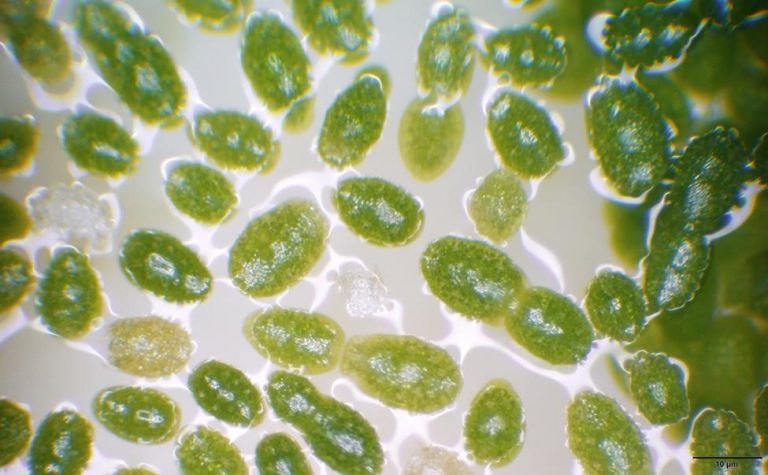
Figure A-196. Wolffia columbiana, watermeal.
Source: Barry Rosen.
Pollens

Figure A-197. Pollen, Barber Pond, RI. Pollen can accumulate and resemble cyanobacteria scums.
Source: Linda Green.

Figure A-198. Pine pollen, Pinus sp., Lake Champlain, VT.
Source: Angela Shambaugh.



Figure A-199. Pine pollen, Pinus sp., Lake Memphremagog, VT.
Source: Walter Medwid.
Pollens – Microscopic Images
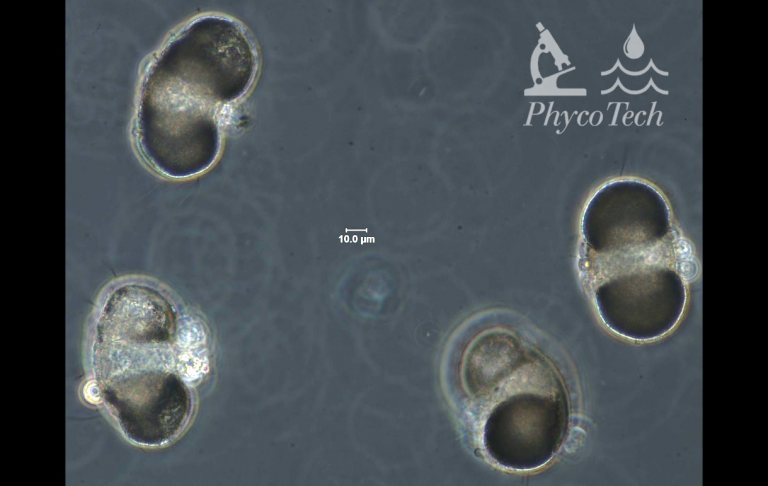
Figure A-200. Pine pollen, Pinus sp., phase.
Source: Ann St. Amand.
Rooted Plants
Chara sp.

Figure A-201. Charophyta, Chara sp., Berrien County, MI. This taxon has a characteristic crumbly, crunchy texture.
Source: Ann St. Amand.

Figure A-202. Charophyta, Chara sp.
Source: Ann St. Amand.

Figure A-203. Charophyta, Chara sp.
Source: Ann St. Amand.
Rooted Macrophytes

Figure A-204. Rooted macrophytes, Kalamazoo County, MI. Rooted macrophytes include a wide variety of aquatic plants that often also support a diverse community of algae and cyanobacteria.
Source: Ann St. Amand.

Figure A-205. Rooted macrophytes, Kalamazoo County, MI.
Source: Ann St. Amand.
Zooplankton Resting Spores (Ephippia)

Figure A-206. Like cyanobacteria, zooplankton resting spores are buoyant and will float.
Source: Angela Shambaugh.

Figure A-207. Surface accumulation of zooplankton resting spores, Lake Champlain, VT.
Source: Pete Stangel.
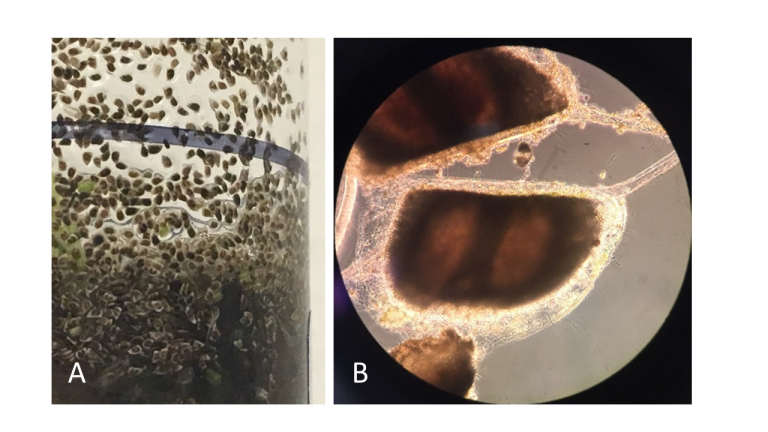
Figure A-208. Zooplankton resting spores lack photosynthetic pigments and have a very uniform appearance. Closer look (A) and appearance under the microscope (B).
Source: Angela Shambaugh.
A-9 Algal Taxonomy
Algal taxonomy is rapidly changing based on a shift from only morphological identification to a polyphasic approach that includes both morphological and genetic characteristics. As more genetic information becomes available, many taxa are being revised into new genera and species. Generally, this split of taxa within genera is helpful and logical, if not a bit cumbersome when trying to compare current to past names. When a genus name changes, the species name often remains the same, except for an ending (for example, Anabaena circinalis to Dolichospermum circinale). Most of the planktonic HCB genera were split based on the production of aerotopes, which are genetically distinct from non-aerotope-forming species. The changes that have been happening over the last couple of decades are based on aerotope presence; location and shape of akinetes in heterocystic cyanobacteria; and reproductive strategies. The best source for checking a current name is the primary literature, but there is also a reasonably up-to-date online source as well: AlgaeBase.org (Guiry and Guiry 2021). We have constructed a short crosswalk table (Table A-1) that includes most of the common HCB taxa. We have included the current name, the old name, and the AlgaeBase link to the current name.
Table A-1. Commonly Accepted and Previous Cyanobacteria Names
A-10 References
CDC. 2021. “Harmful Algal Bloom (HAB)-Associated Illness.” https://www.cdc.gov/habs/index.html.
Guiry, M.D., and G.M. Guiry. 2021. “AlgaeBase. World-wide electronic publication.” National University of Ireland, accessed 08/14/2021. https://www.algaebase.org.
Kannan, Miriam Steinitz, and Nicole Lenca. 2013. “Field guide to algae and other “scums” in ponds, lakes, streams and rivers.” Boone and Kenton County Conservation Districts and the Campbell County Conservation District https://www.townofchapelhill.org/home/showdocument?id=28866.
Komárek, Jiří. 2013. Cyanoprokaryota: III. Teil: Heterocystous genera. Edited by Burkhard Büdel, Georg Gärtner, Lothar Krienitz and Michael Schagerl. Vol. 19(3), Süßwasserflora von Mitteleuropa. Heidleberg: Springer Spekrum.
Komárek, Jiří, and Konstantinos Anagnostidis. 2001. Cyanoprokaryota I. Teil. Chroococcales. Edited by H. Ettl, G. Gartner, J. Heynig and D. Mollenhauer. Vol. 19(1), Süsswasserflora von Mitteleuropa, . Jena: Fischer.
Komárek, Jiří, and Konstantinos Anagnostidis. 2005. Cyanoprokaryota. 2. Teil/2nd Part, 2. Teil/2nd Part. München: Elsevier GmbH.
Komárek, Jiri, Jan Kaštovský, Jan Mares, and Jeffrey Johansen. 2014. “Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach.” Preslia -Praha- 86:295-335.
Otsuka, S, S Suda, S Shibata, H Oyaizu, S Matsumoto, and M M Watanabe. 2001. “A proposal for the unification of five species of the cyanobacterial genus Microcystis Kützing ex Lemmermann 1907 under the rules of the Bacteriological Code.” International Journal of Systematic and Evolutionary Microbiology 51 (3):873-879. doi: https://doi.org/10.1099/00207713-51-3-873.
Rosen, Barry H., and Ann St. Amand. 2015. “Field and Laboratory Guide to Freshwater Cyanobacteria Harmful Algal Blooms for Native American and Alaska Native Communities.” Reston, VA. http://pubs.er.usgs.gov/publication/ofr20151164.
Schmitt, Catherine. 2005. “A Field Guide to Aquatic Phenomena.” University of Maine and Maine Department of Environmental Protection. https://hlcc.org/oldsite/wp-content/uploads/2012/11/Maine-Field-Guide-to-Aquatic-Phenomena.pdf.
USEPA. 2021. “Health Effects from Cyanotoxins.” U.S. Environmental Protection Agency. https://www.epa.gov/cyanohabs/health-effects-cyanotoxins.
Wacklin, Pirjo, Lucien Hoffmann, and Jiri Komárek. 2009. “Nomenclatural validation of the genetically revised cyanobacterial genus Dolichospermum (RALFS ex BORNET et FLAHAULT) comb. nova.” Fottea 9:59-64. doi: 10.5507/fot.2009.005.
Wehr, J. D., R. G. Sheath, and J. P. Kociolet. 2014. Freshwater Algae of North America: Ecology and Classification. 2nd Edition. Boston: Academic Press.