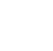1.1 Overview of Cyanobacteria
Cyanobacteria is a bacterial phylum (Stanier 1977), and cyanobacterial species are a common component of the microbial communities found in water or growing at the bottom of oceans, lakes, ponds, rivers, wetlands, and streams across the globe. Cyanobacteria are also found in many terrestrial environments. Cyanobacteria evolved over 2 billion years ago and have adapted to inhabit many environments across the globe. For example, they can grow within rocks in the desert (Huang et al. 2020), have also been found growing without light in rocks deep below the surface of the earth (Puente-Sánchez et al. 2018), and inhabit hot springs that would kill most algae (Ward et al. 1998). In the last decade, the phylum Cyanobacteria was expanded to include the recently discovered nonphotosynthetic group called Melainabacteria, which had previously been thought to be its own distinct phylum (Garcia-Pichel et al. 2020, Soo et al. 2017, Soo et al. 2014). Cyanobacteria are often called algae, a generic term that includes organisms from a variety of taxonomic groups, such as green algae, diatoms, and dinoflagellates (Guiry 2021). However, other algae are eukaryotes, with a nucleus and organelles, which makes cyanobacteria evolutionarily distant and quite distinct from other organisms referred to as algae (Stanier 1977).
Cyanobacterial blooms occur when environmental conditions trigger rapid growth and accumulation of cyanobacterial biomass in a water body (Chorus and Welker 2021, Huisman et al. 2018). Cyanobacterial blooms are also referred to as blue-green algal blooms or harmful algal blooms (HABs). In this document, we use the term harmful cyanobacterial bloom (HCB) specifically to distinguish cyanobacteria from other potentially harmful algae populations in marine and freshwater habitats.
HCBs can occur in many parts of a water body. Planktonic HCBs occur when cyanobacteria dominate the open water of water bodies. The ITRC HCB-1 guidance (ITRC 2021) includes information about planktonic HCBs. In addition to being suspended in the open water, some cyanobacterial species grow attached to surfaces in a water body (Quiblier et al. 2013, Wood et al. 2020). These attached cyanobacteria can grow at the bottom of a water body (benthic zone) but may also be found nearer to the surface growing on submerged vegetation or woody debris (Figure 1-1). Attached cyanobacteria can be found growing on rocks, sediments, wood, and aquatic vegetation in rivers, lakes, ponds, and wetlands. In any of these habitats, benthic cyanobacterial mats can produce and release cyanotoxins into the environment. When cyanobacteria proliferate as attached mats in benthic habitats instead of planktonic blooms, they present unique challenges to evaluating and communicating the public health and environmental risks caused by this less familiar appearance of cyanobacteria.

Figure 1‑1. Images of benthic cyanobacterial mats. A: Microcoleus sp., B: Nostoc sp., C: Anabaena sp., D: detached cyanobacterial and algal mats that have accumulated in a swimming hole in a river.
Source: Keith Bouma-Gregson.
Planktonic HCBs have been documented in the scientific literature for centuries (Codd et al. 2015, Francis 1878, Kirkby 1672). In contrast, benthic HCBs have received more research attention only in the last few decades, and the amount of overall scientific research on benthic HCBs lags behind planktonic HCBs (Quiblier et al. 2013, Wood et al. 2020). Currently, cyanotoxins from benthic HCBs have been reported in many countries across the globe (Figure 1-2). In wadeable streams and nearshore areas of large rivers, lakes, and reservoirs benthic HCBs can create public health and drinking water risks due to the production of cyanotoxins. It is difficult to conclude whether the rise in public health and drinking water concerns with benthic HCBs represents an increase in toxin-producing benthic cyanobacteria in the environment or is the result of increased monitoring, sampling, and understanding of these potential risks. Regardless, in many water bodies, benthic HCBs pose a threat to water quality and require some unique considerations for monitoring and management compared to planktonic HCBs.
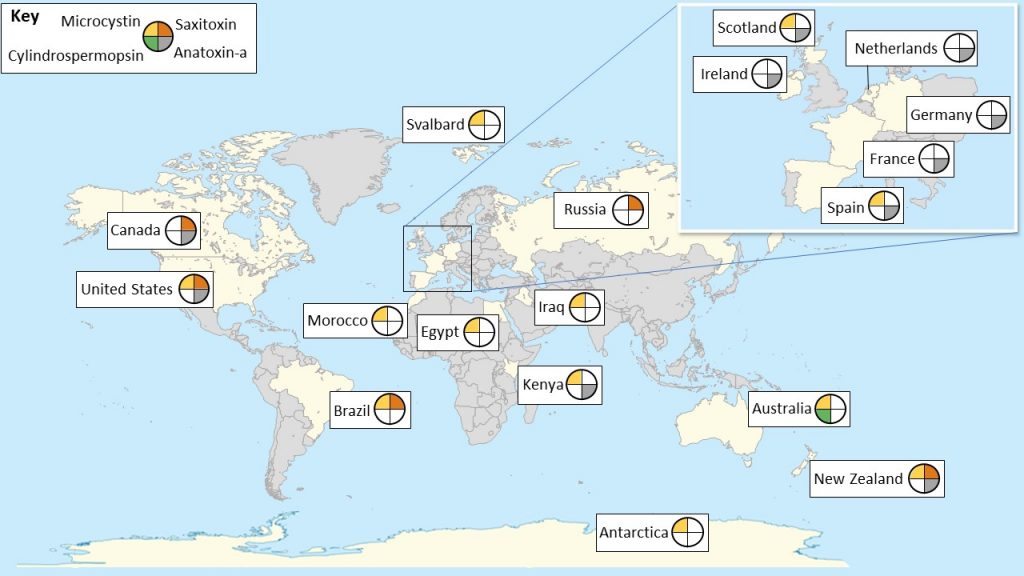
Figure 1‑2. Reported cyanotoxin detections from benthic cyanobacteria across the globe.
Source: Modified from (Wood et al. 2020).
1.2 Scope of Document
The scope of this document is freshwater non-planktonic HCBs that are primarily attached or loosely associated with various surfaces and includes the movement and fate of these mats once they detach and disperse. This document is a companion to the ITRC’s HCB-1 guidance document (ITRC 2021).
The terminology associated with benthic algae and cyanobacteria can be complicated. We will use “benthic cyanobacteria” to mean non-planktonic cyanobacteria that grow attached to, or associated with, surfaces in a water body, often forming macroscopic mats. We recognize that some attached cyanobacterial mats do not grow strictly in the benthic zone of a water body and have chosen to use benthic cyanobacteria to limit our terminology and because most non-planktonic cyanobacteria originate in the benthic zone of a water body. Other terms that are associated with benthic algae and cyanobacteria are periphyton, metaphyton, mats, and biofilm.
This HCB document contains information on benthic (attached) cyanobacteria, monitoring for benthic cyanobacteria, thresholds for cyanotoxins (regardless of source, shared by HCB-1 and HCB-2), strategies for managing and preventing benthic HCBs, and communicating risks and responding to benthic HCBs. We focus on common toxin-producing or nuisance taxa and do not address the entire diversity within the phylum Cyanobacteria. There is a Visual Guide to Cyanobacteria (Appendix A, shared by HCB-1 and HCB-2) to help identify benthic and planktonic HCB taxa, case studies that provide information on how others have handled HCBs (Appendix B, specific for HCB-2), and fact sheets on several HCB management strategies (Appendix C, shared by HCB-1 and HCB-2). The document is designed for people who want to know more about benthic cyanobacteria or need assistance in selecting monitoring, prevention, management, or communication strategies for benthic HCBs.
1.3 Ecology of Attached Cyanobacterial Mats
Cyanobacteria are a diverse phylum that includes many species with unique characteristics. This section will focus on the ecology of documented nuisance and toxin-producing cyanobacterial mat-forming taxa. Cyanobacterial mats can grow in a variety of habitats and environments, and their distribution and abundance are controlled by many environmental factors. The habitats and ecological drivers associated with cyanobacterial mat growth and proliferation will be reviewed in this section.
1.3.1 Water Body Diversity
Cyanobacterial mats can be found in many aquatic habitats where there is enough light to support growth and biomass accumulation (Figure 1-3). In lakes, they can occur in multiple habitats, such as shoreline surfaces, in beds of aquatic plants (macrophytes), and down in deeper waters if the water column is clear. In rivers, mats can grow in faster flowing riffles, as well as slower pool habitats. Cyanobacteria can also grow in wetlands and small ponds. Artificial surfaces, like concrete, steel, or plastic, are suitable for mat growth and shallow artificially lined reservoirs can also host cyanobacterial mats (Izaguirre, Jungblut, and Neilan 2007). Cyanobacterial mats occur in extreme habitats too, such as hot springs.
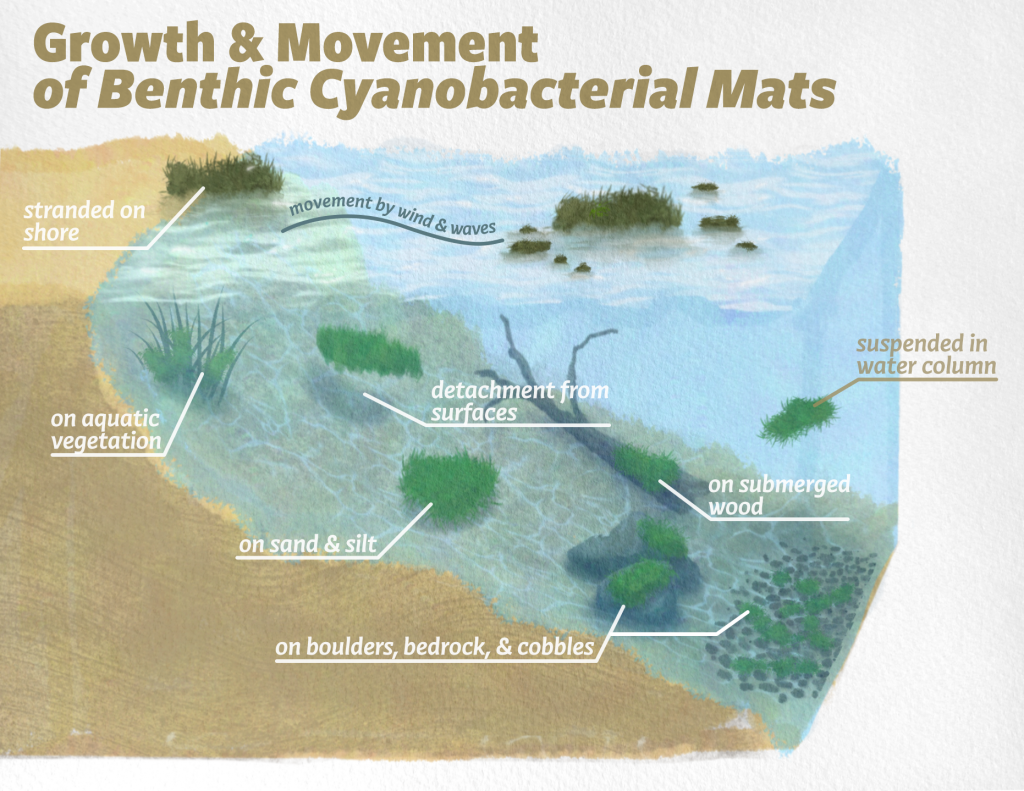
Figure 1‑3. Potential growth habitats and movement of cyanobacterial mats in a water body.
Source: D’yani Wood and Morgan Tarbell.
Because many water bodies with high water quality have clear water, light can strike surfaces at great depths, enabling the growth of attached algae and cyanobacteria. Therefore, attached cyanobacteria can be found in many pristine waters and watersheds with limited human modifications, rather than only occurring in degraded water bodies. For example, cyanobacterial mats are common in many streams and lakes in polar regions (Vincent and Quesada 2012).
1.3.2 Surfaces and Substrates for Growth
Almost all surfaces in aquatic habitats are covered with a layer of attached algae, cyanobacteria, bacteria, and other microorganisms (Figure 1-3). The characteristics of the surfaces influence the formation of the community that develops on the surface (Vadeboncoeur and Steinman 2002). Rocks (boulders, cobbles, and gravel) provide a generally more stable substrate for growth. Finer particles (organic detritus and sediments such as sand, silt, mud) are generally less stable and susceptible to movement and resuspension from current and wave actions. Although finer particles can be unstable, the water in the pore spaces between these particles can have elevated nutrient concentrations and be an important source of nutrients for attached cyanobacteria (Burkholder 1996). Submerged wood is another substrate for attachment. Artificial substrates, such as concrete, plastic, or steel, can also host biofilms and cyanobacterial mats. Cyanobacteria can also grow on aquatic plants or filamentous algae, which can grow up toward the top of the water column where attached cyanobacteria accumulate away from the bottom of a water body. Near the shorelines of lakes, rivers, and streams, a mixture of attached, loosely attached, and detached cyanobacterial mats can accumulate in the sheltered waters within beds of aquatic vegetation and algae (metaphyton), and along shorelines. The characteristics of a particular surface interact with the overall environmental conditions (for example, light, temperature, and nutrients, etc.) to control the types of organisms that grow in that location.
1.3.3 Diversity of Mat-forming Cyanobacteria
Benthic cyanobacterial mats are rarely formed by a single species. A single benthic mat typically comprises multiple cyanobacteria, algae, and other microbial species, in addition to other substances such as detritus and organic molecules (Battin et al. 2016, Quiblier et al. 2013). Despite the diversity within mats, many mats can be dominated by cyanobacteria. The cyanobacteria themselves could be from various species, and a combination of toxic and nontoxic cyanobacteria are frequently observed. Common toxic benthic cyanobacteria are listed in Table 1-1.
Table 1‑1. Common mat-forming cyanobacteria and associated cyanotoxins
Source: Table adapted from SWAMP’s California Freshwater Harmful Algal Bloom Field Guide (SWAMP 2020).
| Taxa | Microcystin | Nodularin | Cylindrospermopsin | Anatoxin-a | Saxitoxins |
| Anabaena | X | X | X | ||
| Anagnostidinema* | X | X | |||
| Cylindrospermum | X | X | |||
| Fischerella | X | ||||
| Geitlerinema | X | X | X | ||
| Hapalosiphon | X | ||||
| Heteroscytonema* | X | ||||
| Iningainema | X | ||||
| Kamptonema* | X | ||||
| Leptolyngbya* | X | ||||
| Microseira wollei* | X | X | |||
| Microcoleus* | X | X | X | X | |
| Nodularia | X | ||||
| Nostoc | X | X | X | ||
| Oscillatoria | X | X | X | ||
| Rivularia | X | ||||
| Scytonema | X | X | |||
| Tolypothrix | X | ||||
| Tychonema* | X |
See Section 2 for more information about dermal cyanotoxins and other secondary metabolites.
There are many different species and genera within the phylum Cyanobacteria. Benthic cyanobacteria of concern tend to be filamentous, with individual cells forming microscopic filaments. These filaments then stick together with extracellular polymeric substances (EPS) to form visible mats. Although planktonic taxa can also be filamentous (for example, Dolichospermum or Aphanizomenon), the most common planktonic HCB species, Microcystis, is unicellular and forms irregular-shaped colonies in the water column. Some genera of cyanobacteria contain species that are predominantly benthic (such as the genus Nostoc); however, other genera (such as Planktothrix (Pancrace et al. 2017) or Nodularia (Lyra et al. 2005) can contain both planktonic and benthic species. Cyanobacterial species with small cells (<2–3 µm) (picocyanobacteria) can also be present in mats. Picocyanobacteria have been understudied historically, due to their small size, but some have been shown to produce cyanotoxins (Śliwińska-Wilczewska et al. 2018) and their importance in many planktonic and benthic aquatic habitats is increasingly recognized (Jasser and Callieri 2016). Similar to planktonic HCBs, there can be ecological and physiological diversity to benthic HCB-forming species. For example, both nitrogen-fixing and non-nitrogen-fixing taxa are common mat formers. Different species are also often associated with different substrates and habitats.
Cyanobacterial taxonomy is rapidly changing from morphological identification based on physical features to an approach that includes both morphological and genetic characteristics. As more genetic information becomes available, many existing taxa are being reorganized into new genera and species (Komárek 2018, 2020). Overall, these changes are helpful and logical for taxonomists; however, it takes time for everyone to fully accept and use the change. The previous taxonomic names are still present in older documents and outreach materials, which can be confusing. There may be no way to be sure that the organism discussed in older materials would now be identified by the new name. Some common mat-forming taxa that have undergone taxonomic revisions include: Lyngbya to Microseira, Phormidium to Microcoleus, and Anabaena with gas vesicles to Dolichospermum, while attached species remain Anabaena. Other taxonomic revisions have occurred, and you can refer to Section A.9 of the Visual Guide for information about other revisions.
In this document, we use the current taxonomic name in most cases; however, where the text refers to an older published document, we use the name found in the source material. We also provide a link to one of the best sources to verify current taxonomy: AlgaeBase.org (Guiry and Guiry 2021).
1.3.4 Environmental Factors that Impact Mat Growth
Environmental conditions constrain or enhance the growth of cyanobacterial mats. Cyanobacteria have evolved to grow under varying light conditions, scavenge nutrients, survive in different habitats to outcompete other microorganisms within a mat, and defend themselves from being eaten by grazers. Out of this complex web of ecological interactions and environmental factors, benthic mats expand, contract, or are held in check.
1.3.4.1 Light
The amount of light influences the growth rates of most cyanobacteria. Photosynthetic cyanobacteria use sunlight to transform carbon dioxide and other nutrients into organic molecules to create and maintain cyanobacterial cells. When light intensity is low, less energy is available and cyanobacterial growth rates decrease.
The intensity and color of light that strikes a submerged cyanobacterial cell is controlled by the molecular properties of water, as well as the concentration and types of dissolved molecules and suspended particles in the water (Kirk 2010, Falkowski and Raven 2007). The amount of available light decreases exponentially with depth as water molecules absorb and scatter light. Dissolved molecules and suspended particles (such as sediments or phytoplankton) also absorb and scatter light. In some turbid water bodies, the light intensity can be reduced by 99% after traveling through the top few meters of the water column. If the water column is too turbid, then there may not be enough light reaching submerged surfaces for benthic mats to proliferate.
Light intensity decreases as it travels through the mat and is absorbed and scattered by cells and substances in the mat (Figure 1-4). Cells that inhabit the lower zones of the mat will be shaded by the cells above them. Many motile taxa will adjust their vertical and horizontal position within the mat to regulate how much light they receive (Biddanda et al. 2015), or to regulate a different environmental factor such as nutrients or oxygen (Garcia-Pichel, Mechling, and Castenholz 1994, Hoiczyk 2000). The color of the light will also change as it travels through the mat because photosynthetic molecules primarily absorb blue, orange, and red light for photosynthesis. Some cyanobacteria have evolved the ability to adapt their photosynthetic apparatus to produce chlorophyll-f (Chen et al. 2010) and absorb the far-red light that penetrates to the deepest layers of the mat (Gan, Shen, and Bryant 2014).
Some cyanobacteria are adapted to low-light conditions and can form mats at the bottom of deep water bodies or shaded habitats. In fact, cyanobacteria are commonly found at the bottom of deep clear lakes (Vadeboncoeur and Power 2017, Wood, Kuhajek, et al. 2012). However, cyanobacterial biomass accrues slowly in these dim environments. Benthic cyanobacteria are shaded as phytoplankton or suspended sediment concentrations increase and prevent light from striking the surfaces at the bottom of the water body. This shading can constrain the growth of cyanobacterial mats to the shallower portions of a turbid water body or a water body with a phytoplankton bloom. Therefore, cyanobacterial mats are often more abundant in oligotrophic water bodies with relatively clear water compared to eutrophic water bodies with high concentrations of suspended particles and nutrients in the water column. In addition, nontoxic filamentous algae have also been increasing in the nearshore waters of oligotrophic lakes (Vadeboncoeur et al. 2021).
1.3.4.2 Nutrients and Carbon
Although cyanobacteria obtain energy from sunlight, they require nutrients from their environment to build the molecules necessary for life. Nitrogen and phosphorus are often the two limiting nutrients, and planktonic cyanobacterial blooms often occur at high nitrogen and phosphorus concentrations (ITRC 2021, Wurtsbaugh, Paerl, and Dodds 2019). However, the optimal nutrient concentrations vary among cyanobacterial species, and planktonic blooms can occur at a variety of concentrations of nitrogen and phosphorus, depending on the taxa forming the bloom (Dolman et al. 2012). Micronutrients (such as iron, calcium, potassium, etc.) can also limit growth (Facey, Apte, and Mitrovic 2019), and in some systems may interact with macronutrients (nitrogen and phosphorus) in controlling the bloom growth rates and density. Access to nutrients is one of the most significant differences between attached and open-water habitats. Cyanobacterial mats can access nutrients from many substrates, such as nutrients leaking out of macrophytes or macro-algae, microorganisms, or nutrients in the pore water of sediments and silt (Figure 1-4). Groundwater infiltrating into a water body first passes through the benthic zone, and nutrients in groundwater can be an important driver of benthic algal and cyanobacterial biomass (Brookfield et al. 2021, Vadeboncoeur et al. 2021). Therefore, unlike planktonic blooms, cyanobacterial mats can proliferate when nutrient concentrations in the water column are low. For example, the growth of Microcoleus and Phormidium mats in rivers can occur when dissolved phosphorus is below 10 μg/L (Wood et al. 2017). Cyanobacterial mats often dominate on substrates in many remote low-nutrient water bodies such as alpine lakes (Mez, Hanselmann, and Preisig 1998) or in the polar regions (Vincent and Quesada 2012).

Figure 1‑4. Conceptual model of some of the physical and chemical factors that control the growth and development of attached cyanobacterial mats.
Source: D’yani Wood and Keith Bouma-Gregson.
Additionally, cyanobacteria can obtain nutrients from organisms or particles within the mat (Lock et al. 1984, Tee et al. 2020). As mats thicken, nutrients that have leaked out of cells or are bound to particles that have become stuck in the mat accumulate and can be recycled within the mat (Figure 1-4). For example, the phosphorus concentration within riverine cyanobacterial mats has been shown to be 300 times higher than in the water column (Wood et al. 2015). How organisms are distributed within the mat is partially controlled by microhabitats formed by the distribution and diffusion of nutrients within the internal structure of the mat.
Last, some cyanobacterial species can obtain nitrogen from the atmosphere. Through a process called nitrogen fixation, cyanobacteria can transform nitrogen gas into ammonia molecules, which can then be used to synthesize amino acids and proteins (Bothe et al. 2010). Not all cyanobacteria can fix nitrogen (Latysheva et al. 2012), but for those that can, this process can alleviate nitrogen limitation in oligotrophic systems. Additionally, some fixed nitrogen will eventually leak out of cells, so other microorganisms often also benefit from the presence of nitrogen fixers in a system. Taken together, the processes described above grant attached cyanobacteria a diverse set of sources and methods to acquire nutrients, which can enable them to access nutrients unavailable to planktonic cyanobacteria.
Although nutrients, such as nitrogen and phosphorus, are essential to many complex molecules necessary for cyanobacterial survival, carbon is the fundamental atomic building block for survival and growth. Cyanobacteria uptake dissolved inorganic carbon in the water and use the energy provided by photosynthesis to break molecular bonds and build new organic molecules, a process called carbon fixation. Some cyanobacteria can grow slowly using sugars, instead of carbon dioxide, as a carbon source (Stanier and Cohen-Bazire 1977), but this is not thought to contribute to HCBs. When HCBs obtain high biomass, carbon concentrations can be drawn down by the HCB, which limits growth due to the chemistry of inorganic carbon and the enzymes used in carbon fixation. To overcome these constraints, cyanobacteria and algae evolved carbon-concentrating mechanisms (CCMs) (Giordano, Beardall, and Raven 2005, Price et al. 2008). CCMs are diverse and there are many variations in how they function across both algae and cyanobacteria. In cyanobacteria, CCMs include protein structures—carboxysomes that form microcompartments inside the cell where carbon can be concentrated. With carbon concentrated in the carboxysome, carbon fixation occurs more efficiently, and cyanobacterial growth can be sustained. In planktonic Microcystis blooms, environmental conditions can select for strains with different types of CCMs (Sandrini et al. 2016). When algal and cyanobacterial biomass is high, the competition for inorganic carbon and the ability to efficiently fix carbon plays a role in determining which species and strains dominate an algal community (Beardall and Raven 2017, Raven et al. 2011).
1.3.4.3 Water Velocity
The velocity of water flowing over a surface influences the community composition and overall biomass of attached cyanobacteria or algae. Turbulence and boundary layers are properties of flowing water that influence the microenvironment surrounding attached mats (Vogel 1996). Turbulence is disordered flow consisting of swirling eddies, which can move molecules throughout the water column. However, as the water gets closer to a submerged surface, such as a rock or boulder, the friction between the surface and the water causes the water velocity to slow. Right next to the submerged surface the water velocity is zero. The boundary layer is the transition zone where velocity decreases from the speed of the free-flowing open water high above the surface to a velocity of zero at the submerged surface. As the water velocity decreases, the flow becomes more ordered and parallel. Without the swirling eddies, molecules travel slowly in directions other than the overall flow direction.
The characteristics of the boundary layer control the delivery rate of molecules from the water body into the mat. As flows become faster and more turbulent, nutrient limitation can be alleviated as the thickness of the boundary layer decreases (Figure 1-4) and more dissolved or suspended particles in the water column are delivered to attached cyanobacteria. However, as flow increases, drag on attached cells also increases. If the force of drag is greater than the attachment strength, then cells will be pulled off a surface. Therefore, velocity results in two counteracting processes of delivering materials to (subsidies) and increasing drag on (stress) attached cyanobacteria and algal mats (Biggs, Goring, and Nikora 1998).
Different types of benthic cyanobacteria are suited to different water flow conditions. The attachment strength and resistance to drag vary with growth form and the physiology of specific species (Biggs and Thomsen 1995). Thin mats and biofilms, with low drag, are usually found in faster flowing waters. Even centimeter-scale variation in turbulence and velocity has been shown to affect the composition of diatom communities (Stevenson 1983). As mats thicken, drag increases, which can limit thick mats or long filamentous algae to habitats with lower water velocities. An experiment in New Zealand artificially increased flow in experimental units and shifted the community from filamentous green algae to thinner cyanobacterial mats (Hart et al. 2013).
Many aquatic systems are dynamic, and fluctuations in flow velocity can disturb communities of attached cyanobacteria. In rivers, spring and summer rain events that cause short-term flow increases can detach large amounts of biomass from substrates. Shoreline areas of lakes can be disturbed by waves from high winds or boat wakes, detaching cyanobacteria and algae from their substrates and washing it on shore. Ice can also scour away benthic mats. The strength, duration, and timing of these disturbances will affect how the cyanobacterial community responds, and whether the same taxa or mat can recover, or whether a new community will replace the old (Power and Stewart 1987, Sousa 1984).
1.3.4.4 Temperature
Cyanobacterial growth rates change with temperature. The optimal growth temperature for many cyanobacteria is warmer than for diatoms, while green algae and cyanobacteria have similar optimal growth temperatures (Lürling et al. 2013, Paerl, Hall, and Calandrino 2011). Importantly, interactions between temperature and other factors such as nutrients or grazing complicate interpretation of the effect of increased temperature on the overall concentration of cyanobacteria in a water body (Richardson et al. 2018, Rigosi et al. 2014, Schulhof et al. 2019). The warmest environmental temperatures do not always result in the highest cyanobacterial biomass. Cold temperatures do not prevent the formation of cyanobacterial mats; they can be found in high latitudes and high elevations. In summary, many cyanobacterial taxa can tolerate broad temperature ranges (Tang, Tremblay, and Vincent 1997), with growth rates generally decreasing as temperatures drop.
1.3.4.5 Grazing
Cyanobacteria and algae form the base of food webs and are consumed by many other microbes, insects, fish, and other organisms (Vadeboncoeur and Power 2017). The grazing pressure can strongly control the distribution and biomass of attached algae and cyanobacteria in aquatic systems (Hart 1992, Power, Stewart, and Matthews 1988). Decreases in grazer density or shifts in the community composition of grazers have been hypothesized as a driver of increases of benthic cyanobacteria and algal biomass in lakes (Vadeboncoeur et al. 2021). However, the context of the habitat and the timing of grazing prevent broad generalizations about the impact of grazing on the community composition or overall biomass of attached algae and cyanobacteria (Steinman 1996). Most of the research on cyanobacterial grazing has been focused on planktonic species being consumed by zooplankton and grazing of benthic mats in marine systems. Few studies have focused on the grazer interactions with toxin-producing freshwater cyanobacterial mats.
The outcome of interactions between grazers and attached algae and cyanobacteria depends on the traits of these organisms and the timing of their interactions (Steinman 1996). Because they lack certain fatty acids, cyanobacteria are generally considered less nutritious than other algae. Additionally, the shape and colonial form of some cyanobacterial taxa make them more difficult to consume than diatoms (Ger, Hansson, and Lürling 2014). Therefore, some grazers can experience decreased growth or fitness when consuming only cyanobacteria. Species that are more strongly attached to surfaces are also more resistant to grazing. Grazers can consume the upper canopy of attached biofilms, while the lower sections of the biofilm are more resistant to consumption (Vadeboncoeur and Power 2017). As grazers consume other more easily ingestible taxa the firmly attached species will often increase in abundance.
Many grazing species are selective in which algae they consume (Moore 1975). For example, a caddisfly species was shown to selectively remove cyanobacteria from cobbles to prevent the cyanobacteria from overgrowing its preferred food source of diatoms and green algae (Hart 1985). Experiments with snails have observed decreases in cyanobacterial biomass when snails are present, which impacted the successional development of the attached algal and cyanobacterial communities (Groendahl and Fink 2017, Tuchman and Stevenson 1991).
Importantly, the timing of grazing pressure and mat development must be considered when predicting the outcome of grazer-cyanobacterial interactions. Filamentous taxa may be grazed when they are short, but can escape grazing by becoming too long for grazers to consume (Dudley and D’Antonio 1991, Seymour Brown 1960). The timing of grazing is a primary factor controlling how consumption will transform the diversity and biomass of an algal and cyanobacterial mat.
1.3.4.6 Interactions with Other Plants and Algae
In addition to grazing, cyanobacteria interact with micro- and macroorganisms in other positive or negative ways. There is competition for resources between microorganisms as cyanobacteria and algae try to acquire light, nutrients, and space to survive and grow. To remain successful in this competitive environment, many organisms produce allelopathic chemicals, compounds to inhibit or promote the fitness and growth of neighboring cells and species (Gross 2003). Many cyanobacteria have been shown to produce these compounds that inhibit other algal taxa (Leão et al. 2010). There is also evidence that aquatic vegetation can produce allelochemicals to prevent the attachment of algae and cyanobacteria on their structures (Gross 1999, Hilt 2006); however, the accumulation of biofilms on most aquatic vegetation indicates that allelochemicals are not effective at completely preventing colonization by other organisms. Because allelopathic compounds can have different modes of action and be produced at low environmental concentrations, it can be difficult to isolate the role of allelopathic compounds in structuring algal and cyanobacterial communities. However, scientists are increasingly acknowledging the role of chemical interactions in determining the outcome of interactions between species. Overall, there are many competitive and inhibitory interactions between cyanobacteria and other micro- and macroorganisms, which together partially control the distribution and abundance of cyanobacterial mats in aquatic habitats.
Cyanobacterial interactions can also be positive for the cyanobacterium or other organisms. Within a microbial mat a complex assemblage of bacteria performs many functions that benefit cyanobacteria and algae, such as reducing the concentration of toxic waste compounds or increasing the concentration of micro- or macronutrients in a mat. Additionally, many cyanobacteria occur in symbiosis with other organisms, such as plants, lichens, and algae. For example, species in the genus Nostoc have a symbiosis with the water fern Azolla, and nitrogen-fixing cyanobacteria live inside several genera of diatoms (Foster et al. 2011, Nakayama and Inagaki 2017).
1.3.5 Life Cycle of Cyanobacterial Mats
Mats usually have a life cycle that includes the stages of colonization, growth and expansion, and senescence and detachment (McAllister, Wood, and Hawes 2016, Wood et al. 2020). During the colonization stage, microscopic cells are attaching to surfaces. The success of establishment depends on the characteristics of the surface and the characteristics of the microorganisms that currently inhabit that surface (Figure 1-5). Once microscopically established on a mat, the cells will begin to divide, and the mat thickens and expands. Many mat-forming taxa are motile (both diatoms and cyanobacteria) and can glide along surfaces or within the mat (Castenholz 1967). This facilitates horizontal and vertical expansion of the mat. In many cases the microscopic cells will grow and expand until the mat becomes visible in the bottom of the water body.
The last stage of the life cycle is senescence and detachment. As the mats age, basal cells may weaken due to shading from cells above, unfavorable conditions within the mat matrix, or infections from bacteriophage. As cells weaken, pieces of the mat will begin to detach. Alternatively, a healthy mat may detach if the mat has grown too large and buoyancy or drag forces pull it off the substrate. A common mechanism for mat detachment is the accumulation of oxygen bubbles within mats. The oxygen is produced by photosynthesis and gets trapped and forms bubbles in the mucilaginous EPS matrix of the mat (Bosak et al. 2010). As photosynthesis increases the amount of oxygen bubbles within the mat matrix, the buoyancy forces can exceed the attachment strength and pull a mat off the substrate. Once detached, mats will rise to the surface and be transported by wind and currents to new locations. Floating mats can accumulate in eddies or may be washed on shore by wind and currents. Another mechanism for detachment is scouring from wave action, ice, or increasing river flow (for example, flood pulses from summer thunderstorms). These hydrologic mechanisms may detach mats before they have reached maximum growth and begun senescing. In New Zealand, high-flow events in the summer are correlated with lower benthic cyanobacterial coverage (Heath et al. 2013, Wood et al. 2017). Last, easier mat detachment may occur when environmental and ecological conditions change and no longer favor cyanobacterial mats. Eventually, whether attached or detached, cyanobacterial cells will respond to changing environmental conditions by entering a resting phase and ceasing growth. Some taxa produce resting cells called akinetes, while for other taxa cells simply go dormant in the sediment until environmental conditions trigger growth again.
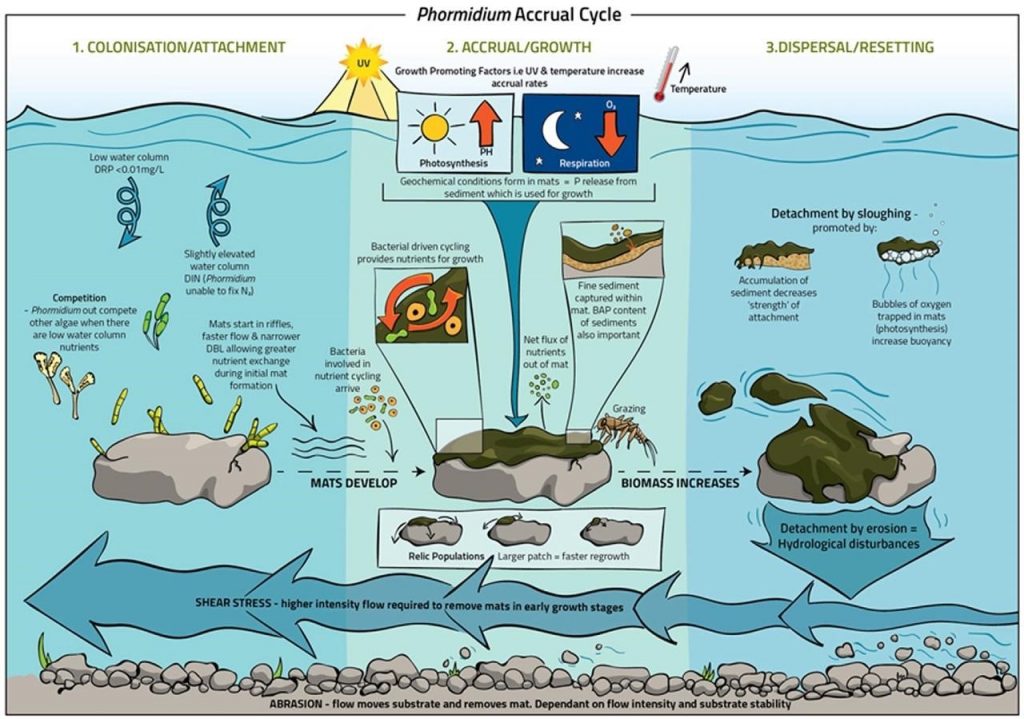
Figure 1‑5. Mat life cycle.
Source: Wood et al. (2015).
1.4 Health, Environmental, and Economic Impacts
1.4.1 Cyanotoxins and Health
Cyanobacteria can produce molecules, called cyanotoxins, that can cause serious health effects in people (Chorus and Welker 2021, Codd et al. 2020). Direct exposure to cyanotoxins may occur when you consume drinking water contaminated by HCBs, eat cyanotoxin-contaminated fish or shellfish, accidentally swallow cyanotoxin-contaminated water during swimming, or breathe in aerosolized cyanotoxins in water spray or mist (Backer et al. 2010, Carmichael 2001, Hilborn et al. 2014, Plaas and Paerl 2021).
There are hundreds of cyanotoxin compounds (Merel et al. 2013, Roy-Lachapelle et al. 2019). Whether cyanotoxins come from planktonic HCBs or benthic HCBs, cyanotoxins can affect human, animal, and aquatic health. Cyanotoxins can be grouped by their target of toxicity, with major groups including hepatotoxins (liver toxins), neurotoxins (nerve toxins), and dermatoxins (skin toxins). The concentration and potency of cyanotoxins in the water or mat biomass can influence the extent of the toxic effect. A summary of the types of cyanotoxins produced by common benthic HCB taxa is shown in Table 1-1. More detailed information on these cyanotoxins, health impacts, and toxicity thresholds is available in Section 2.
Both planktonic and benthic HCBs can produce cyanotoxins. Although the means of cyanotoxin release, the potential dose, and the route of human or animal exposure may differ from planktonic to benthic blooms, the specific cyanotoxins produced by either type of bloom have the same toxicity and potential adverse effects. For example, ingestion of microcystin from a planktonic bloom has the same potential adverse health effect in humans as ingestion of microcystin from a benthic bloom. However, the dose and means of exposure could potentially differ, as a planktonic bloom exposure might be via drinking water and benthic bloom exposure might be via unintentional ingestion of bits of floating biomass.
See Section 2 for more information on cyanotoxins produced by planktonic and benthic HCBs.
1.4.2 Environmental Impacts
The impact of cyanobacterial mats on food webs and ecosystems is complex. Benthic cyanobacterial mats can be integral parts of some aquatic ecosystems. Other organisms are frequently found inhabiting cyanobacterial mats, but it is uncertain if cyanobacterial cells are being consumed by organisms inhabiting mats or if the mats are used as a refuge (Lévesque, Cattaneo, and Hudon 2015). Benthic HCBs also alter the river habitat for invertebrates that inhabit benthic habitats. It is not well understood how benthic proliferations of mats can impact the growth and survival of benthic macroinvertebrates.
The impact of cyanotoxins on benthic macroinvertebrates is variable. A common New Zealand mayfly (Deleatidium spp.) did not exhibit mortality to dissolved anatoxin-a even at very high anatoxin-a concentrations (300–600 µg/L), suggesting that it may be relatively insensitive to environmentally relevant concentrations of anatoxin-a in New Zealand rivers (Kelly et al. 2020). However, a study in California found median lethal anatoxin-a concentrations of <1 µg/L for Hyalella sp., Chironomus sp., and Ceriodaphnia sp. (Anderson et al. 2018). A study on New Zealand crayfish showed that crayfish consumed benthic cyanobacteria, and crayfish tail tissue contained low concentrations of nodularin (Wood, Phillips, et al. 2012).
1.5 Understanding Your Water Body and Developing an HCB Management Plan
1.5.1 Role of a Management Plan
It is important to know your water body’s historical and current water quality condition when managing for cyanobacteria. A good management plan documents the condition of the water body and its watershed, identifies relevant data sources, highlights potential HCB drivers and contributing factors, and identifies uses and endpoints of value. These drivers and endpoints may be different if a planktonic or benthic HCB is impacting a water body. HCB management plans summarize a current understanding of the water quality in a specific water body and identify knowledge and data gaps. This information is important when considering management strategies to address the problem.
Currently there are no management plan frameworks specific to benthic HCBs; however, planktonic HCB management plans are available from North American Lake Management Society (NALMS, NALMS 2021) and the U.S. Environmental Protection Agency (USEPA (2008, 2013). A management plan focused on cyanobacteria that includes both benthic and planktonic may be part of a larger watershed plan, a drinking water source protection plan, or a larger nutrient management plan such as a total maximum daily load (TMDL).
1.5.2 Key Components of an HCB Management Plan
Federal regulations require states and some tribal authorities to characterize water bodies by the type of use they support, such as fishing, shell-fishing, recreation, public water system (PWS), agriculture, industry, and navigation. To protect these designated uses, a state, tribe, or territory establishes specific quantitative or qualitative guidelines known as water quality standards (WQS) that outline acceptable levels for pollutants. It is important to be familiar with the WQS in your state as you identify the goals you wish to achieve through HCB management actions.
Your management plan should summarize and analyze available data and identify data gaps. All comprehensive plans should include considerations for benthic and planktonic forms of HCBs. To accomplish this, considerations for benthic cyanobacteria need to be added to existing planktonic HCB plans or incorporated from the beginning when starting a new plan from scratch.
For HCBs, many different types of data are important to incorporate in your planning. ITRC’s HCB-1 guidance (ITRC 2021) identified nutrients, watershed land use, water body characteristics, weather, food web structure, and previous HCB occurrences as important types of information to integrate into an HCB management plan. Additional components that should be considered for benthic HCBs are the presence and abundance of aquatic plants. These can compete for nutrients and light and may affect water movement, and treatment for aquatic weeds may directly or indirectly affect cyanobacteria (benthic and/or planktonic).
Once you have evaluated the available data, characterized your water body, and outlined the changes you would like to achieve, you can identify strategies that will help you achieve your goals. As strategies are put in place, it is important to continue to monitor and collect data to evaluate how well your strategies are working. Periodically reviewing monitoring data will help you determine if you are reaching your goals. Your HCB management plan ties this all together and sets relevant milestones.
1.5.3 Economic Impacts
HCBs have a multifaceted impact to the U.S. economy caused by higher drinking water treatment costs (monitoring, plant operations, capital investment, communications), loss of livestock and crops, loss of recreational or tourism revenue, undiagnosed health effects, and lower prices for real estate along waterfront properties due to unsightly conditions and foul odor. Some impacts cannot be easily evaluated (for example, undiagnosed health effects and agricultural losses), and we may not fully understand how frequent severe HCBs affect local economies. There are surprisingly few peer-reviewed publications (for example, Baker et al. 2001) documenting HCB economic impacts, and none that focus on benthic HCBs.
A few examples of economic impacts of benthic HCBs can be found. In 2000, a benthic Phormidium bloom in South Australia cost the water supplier $0.5 million Australian dollars to handle the incident, and there were additional losses in tourist revenue (Baker et al. 2001). In 2015, a dog death in Northern California’s Russian River was attributed to cyanotoxin poisoning (Moore 2015). This incident occurred the week before Labor Day and led the Sonoma County Public Health Department to close the river to recreation during the holiday weekend. This had a negative impact on many businesses and the tourism industry associated with the river (Appendix B). In 2020, a benthic HCB–related dog death occurred in Zion National Park in Utah. This led to extensive monitoring to assess the risk to park visitors and downstream agricultural and livestock production. This all had economic impacts on state and federal agencies, the agriculture industry, and the tourism industry in the region (Appendix B).
1.5.3.1 Impacts to Drinking Water Systems
The full impact of benthic cyanobacteria to drinking water systems is uncertain. The production of taste and odor compounds by cyanobacterial mats is the most common reason drinking water systems have management plans for cyanobacterial mats. Drinking water providers less frequently monitor or plan for cyanotoxins produced by cyanobacterial mats. Because cyanotoxins produced by benthic cyanobacteria often remain in the mats, collecting water column samples to test for benthic cyanobacterial toxins may not detect cyanotoxins, even if concentrations within the mats are very high. Mats may also create taste and odor and biofouling issues if they grow and clog intake or conveyance structures. Please refer to ITRC HCB-1 Section 3.2.3.1 Impacts to Public Drinking Water Systems and 3.2.4 Regulatory Requirements for Recreation and Drinking Water for further information (ITRC 2021). USEPA released a document in 2015, “Recommendations for Public Water Systems to Manage Cyanotoxins in Drinking Water,” that may help some PWSs understand what management plans they need (USEPA 2015g).