Cyanotoxins can be produced by planktonic and benthic cyanobacteria. Because the cyanotoxin classes produced by both planktonic and benthic cyanobacteria are overlapping, information presented in this section applies to both planktonic and benthic HCBs. This section covers cyanotoxin exposure routes, health impacts, classes of cyanotoxins, distribution in the environment, and toxicity thresholds. If you want to navigate directly to cyanotoxin thresholds for humans or domestic animals, you can simply click on the appropriate image on Figure 2-1 below.

Figure 2‑1. Cyanotoxin Thresholds.
2.1 Exposure Routes to Cyanotoxins
Exposure to cyanotoxins can occur by ingestion, inhalation, or direct exposure to the skin. The most common routes of exposure are ingestion of water containing cyanotoxins or toxin-containing cyanobacteria cells, either through drinking water or recreational activity in water. Swimming, playing sports, or boating on water containing cyanotoxins or cyanobacteria can result in exposure via ingestion, inhalation of mists, or direct contact. Pieces of benthic algal mats are sometimes present in the water column, and ingestion of these mat pieces can also be a source of exposure. Consumption of fish or other foods from contaminated water might also result in exposure. The concentration of cyanotoxins in the water or biomass and the length of time of the exposure can influence the type and severity of any resulting health effects.
2.2 Health Impacts of Cyanotoxins
Case studies have examined reports of human and animal illnesses related to cyanotoxin exposure, and the Centers for Disease Control and Prevention (CDC) is now tracking those reports. Information about the CDC program is available from Harmful Algal Blooms–Associated Illness site (CDC 2021b). The information below relates to cyanotoxin exposure in general and does not necessarily pertain only to benthic HCBs. The cyanotoxins reported most often in the above incidents were microcystins followed by anatoxin-a (Roberts et al. 2020).
2.2.1 Effects of Cyanotoxins on Human Health
Information about adverse human health effects related to cyanotoxin exposure, particularly for benthic HCBs, is limited. There have been no human deaths confirmed due to recreational exposure to cyanotoxins in freshwater, but there have been illness outbreaks. For example, in the United States between 2016 and 2018, the CDC reports that there were 389 human health cases associated with 73 HCBs recorded in 18 states, with 51% of these cases related to one-time exposures at a lake in Utah (Roberts et al. 2020). The most commonly reported symptoms were gastrointestinal (67%), lethargy (43%), and dermatological (27%), as well as reports of headache and fever (Roberts et al. 2020). Nearly 40% of the human illnesses reported occurred in children under age 18. The majority of reports were received from June to September (Roberts et al. 2020).
2.2.2 Effects of Cyanotoxins on Animals
Illnesses and deaths of animals have also been reported, especially in dogs (Backer et al. 2013) and in livestock (Dreher et al. 2019). In 42 HCB events in 18 U.S. states during 2016 to 2018, there were 369 animal deaths reported among 413 cases of animal illnesses (Roberts et al. 2020). Of these, 52 of the illnesses were companion animals, including 50 dogs (Roberts et al. 2020).
2.2.3 Effects of Cyanotoxins on Aquatic Life
Wood et al. (2020) reported that few studies have looked at the effects of cyanotoxins on benthic organisms. These studies looked at extracts of microcystin and anatoxin-a from planktonic cyanobacteria on the sediment-dwelling midge Chironomus and found that the extracts were more toxic than purified cyanotoxins. The conclusion based on the study by Toporowska and Pawlik-Skowrońska (2014) was that compounds produced by cyanobacteria are likely to have a negative effect on some aquatic organisms. Anderson et al. (2018) showed that crude extracts containing anatoxin-a from the benthic cyanobacteria Phormidium showed significant mortality in both benthic organisms (midges and amphipods) and the planktonic organism Ceriodaphnia dubia. Most of the current aquatic ecotoxicity information is based on acute, short-term exposures to microcystins with more limited information on other cyanotoxin classes (Mehinto et al. 2021).
Bioaccumulation of cyanotoxins in aquatic organisms has been observed, particularly for microcystins (Chorus and Welker 2021). Banerjee et al. (2021) conducted a review of cyanotoxin effects on teleost fish and noted the evidence of bioaccumulation of cyanotoxins in fish species. Kelly et al. (2020) showed that high concentrations of purified anatoxin-a accumulated in a mayfly. They suggested that the possibility of trophic transfer of anatoxins should be investigated. Wood, Phillips, et al. (2012) found that nodularin from benthic mats accumulates in the hepatopancreas and tail tissue of the crayfish. Colas, Duval, and Marie (2019) found that fish collected from French rivers during benthic cyanobacterial blooms had anatoxins in their muscle, gut, and encephalon.
Cyanobacteria exhibit defense strategies to reduce grazing by aquatic organisms (such as zooplankton and macroinvertebrates). In a review of grazing resistance, Lürling (2021) presented instances of cyanobacteria forming large colonies (such as Microcystis) and flakes or bundles of filaments (like Aphanizomenon) that impede grazing or exceed critical dimensions to be consumed. In addition to poor palatability and grazing inhibition, cyanobacteria are of lower nutritional value and potentially toxic to zooplankton, which ultimately reduce vitality rates (such as lower growth and reproduction or increased mortality) of consumers (Moustaka-Gouni and Sommer 2020). Some unpublished information indicates that filamentous cyanobacteria may impede mayfly survival due to either interference with food uptake or physical effects on its ability to live in filamentous cyanobacteria (James Lazorchak, personal communication).
2.3 Overview of Cyanotoxin Classes
Cyanotoxins are secondary metabolites produced by cyanobacteria that could result in adverse health effects in humans, animals, and other aquatic life after exposure through ingestion, inhalation, or contact with water or biomass containing the cyanotoxins. The concentration and potency of cyanotoxins at the time of exposure can influence the extent of the toxic effect.
A summary of the types of cyanotoxins is presented in Table 2-1 below and in Table 3.1 of HCB-1 (ITRC 2021). Cyanotoxins are typically grouped by their dominant target of toxicity in humans and animals as shown in Table 2-1. Concentrations of chemicals that act by the same mode of action are typically summed (assuming additive toxicity) and the overall total is compared to the appropriate threshold. If sufficient information is available to develop toxicity equivalency factors (TEFs) for individual structural variants relative to a reference chemical, then each individual structural variant would be multiplied by that TEF and the sum of overall cyanotoxin equivalents would be compared to the threshold. More details are provided for each cyanotoxin class below.
Table 2‑1. Human and animal health impacts from cyanotoxins and compounds
Source: Adapted in part from Sanseverino et al. (2016) and (Fiore et al. 2020)
| Compound Classification | Cyanotoxin*/Irritant | Main Target Organ | Effects |
| Hepatotoxins | Microcystin | Liver | Diarrhea, vomiting, weakness, liver inflammation, liver hemorrhage, pneumonia, dermatitis |
| Nodularin | Liver | Diarrhea, vomiting, weakness, liver inflammation, liver hemorrhage, pneumonia, dermatitis | |
| Cylindrospermopsin | Liver and kidney | Diarrhea, vomiting, nausea, gastroenteritis, liver inflammation, liver hemorrhage, pneumonia, dermatitis, kidney damage, headache | |
| Neurotoxins | Anatoxin-a | Nervous system | Muscle twitching, burning, numbness, drowsiness, salivation, respiratory paralysis leading to death |
| Guanitoxin -formerly anatoxin-a(S) | Nervous system | Salivation, convulsions, muscle fatigue, and respiratory arrest | |
| Saxitoxins | Nervous system | Muscle twitching, burning, numbness, drowsiness, headache, vertigo, respiratory paralysis leading to death | |
| Β-methylamino-L-alanine (BMAA) | Nervous system | Chronic exposure associated with neurodegenerative disease reported by some studies; scientific discussion is ongoing | |
| Aetokthonotoxin | Nervous system | Avian vacuolar myelinopathy | |
| Dermatoxins and Skin-Irritating Compounds | Aplysiatoxin | Skin | Skin irritation, upper respiratory irritation |
| Lyngbyatoxin | Skin | Skin and eye irritation, respiratory problems | |
| Lipopolysaccharide | Skin | Skin and eye irritation, headache, allergy, upper respiratory irritation, fever |
Cyanobacteria genera associated with cyanotoxins and irritants are listed in the Visual Guide and SWAMP (2020).
2.3.1 Hepatotoxins
Hepatotoxins cause damage to the liver, often by disrupting the liver cells. Three hepatotoxins are briefly described below, including the microcystins, nodularins, and cylindrospermopsins. All three can be produced by benthic or planktonic forms of HCBs. Microcystins are mainly contained within cell walls in healthy cells of cyanobacteria, but they are released in a dissolved form once a cell is lysed.
2.3.1.1 Microcystins and Nodularins
Two types of cyanobacterial hepatotoxins are microcystins and nodularins, which have similar chemical and toxicological properties. Microcystins are globally distributed and found in a wide range of aquatic environments. Nodularins are often found in an estuarine environment. These cyanotoxins are most often produced by species of planktonic cyanobacteria. However, there are some genera, such as Anabaena, Nostoc, and Phormidium, that produce microcystin in benthic forms (Chorus and Welker 2021, WHO 2020c). The specific structural variants of microcystin produced by benthic cyanobacteria taxa are less studied than those of planktonic cyanobacteria.
Microcystins affect the liver by causing breakdown of the liver cells after uptake (Chorus and Welker 2021). Microcystins have been the known or suspected cause of several outbreaks related to drinking water consumption or recreational settings, though no outbreaks have been specifically tied to benthic HCBs (WHO 2020c). Examples of health effects experienced are gastrointestinal symptoms, kidney and liver damage, and dermal rashes (WHO 2020c). More severe impacts, including death, have occurred when microcystin-contaminated water was used for dialysis (Azevedo et al. 2002, Pouria et al. 1998).
Nodularins can induce liver hemorrhage (Pearson et al. 2010), and illnesses have been reported, particularly in animals. No outbreaks in freshwater associated with benthic blooms were found.
Microcystins and nodularins have the following characteristics:
- Cyclic peptide chemical structure with a shared L-amino acid, specifically ADDA (van Apeldoorn et al. 2007).
- Water soluble.
- Stable (Boiling will not degrade them or make the water safe to drink.)
- Release: Microcystins and nodularians release to water when cells senesce, die, and lyse (Chorus and Welker 2021).
- Degradation of cyanotoxin:
- Microcystins have a half-life in water of about 1 week in ambient waters. More acidic water may increase rate of biodegradation (WHO 2003).
- Nodularins degrade quickly in sunlight (O’Neil et al. 2012). Some studies report complete degradation in less than 30 days (Edwards et al. 2008).
- Bioaccumulation:
- Both toxin classes generally accumulate in internal organs, particularly the liver, and to a lesser extent, in muscle tissue (Chorus and Welker 2021, Testai, Buratti, et al. 2016).
- Microcystins have water to fish tissue bioaccumulation factors ranging from 14 to 2,409 and show a range from low to very high accumulation in aquatic organisms (National Center for Biotechnology Information 2021b).
- Nodularins may accumulate in seafood (Engström-Öst et al. 2002).
- Structural variants: Microcystin has more than 250. Nodularin has 10 identified as of 2021 (Chorus and Welker 2021). Analytical standards are available only for a small subset of these structural variants; others may be identified qualitatively.
As noted above, because microcystins and nodularins both act by a similar mechanism of inhibiting protein phosphatases, they are typically assumed to have additive toxicity. Therefore, the sum total concentration of both microcystins and nodularins would be compared to the toxicity threshold. A common analytical method, the ADDA enzyme-linked immunosorbent assay (ELISA), binds to the shared ADDA portion of any microcystins or nodularins present in the sample, so the reported result is applicable. If individual variants of microcystins or nodularins are measured, such as by liquid chromatography–tandem mass spectrometry (LC-MS/MS), then the concentrations of individual variants are generally summed, and the overall total would be comparable to the toxicity threshold. Recent studies are evaluating the relative toxicity of individual microcystin structural variants (Chernoff et al. 2021, Chernoff et al. 2020), but TEFs have not yet been adopted and may vary by type of organism and exposure route. The presence of structural variants that are not quantified by LC-MS/MS due to the lack of analytical standards could result in a sum of all individually measured structural variants that underestimates the actual total microcystins/nodularins present in the sample. Therefore, some states rely more on ADDA ELISA results, rather than potentially underestimating the contribution of structural variants not quantified with LC-MS/MS.
2.3.1.2 Cylindrospermopsins
Another type of hepatotoxin produced by cyanobacteria is cylindrospermopsin. Cylindrospermopsin is often released from viable cells. Notable cyanobacteria strains that produce cylindrospermopsin are Raphidiopsis (previously Cylindrospermopsis) raciborskii, Aphanizomenon flos-aquae, Aphanizomenon gracile, Aphanizomenon ovalisporum, Umezakia natans, Dolichospermum (previously Anabaena) bergii, Dolichospermum lapponica, Dolichospermum planctonica, Lyngbya wollei, Rhaphidiopsis curvata, and rhaphidiopsis mediterranea (USEPA 2021h). Benthic producers of cylindrospermopsin are Microcoleus (formerly Phormidium; Gaget, Humpage, et al. 2017, in SWAMP 2020), Microseira (formerly Lyngbya), and Oscillatoria (WHO 2020b).
Cylindrospermopsins have the following characteristics:
- Alkaloid chemical structure (USEPA 2019).
- Water soluble.
- Stable (Boiling will not degrade them or make the water safe to drink.)
- Release: Cylindrospermopsins may be released from intact cells into water (Chorus and Welker 2021).
- Degradation of extracellular/dissolved cyanotoxin: Cylindrospermopsins have an initial lag period followed by half-lives of 2–4 days.
- Bioaccumulation: Cylindrospermopsins have low levels of bioaccumulation in some species of animals and plants (Chorus and Welker 2021).
- Four structural variants (Chorus and Welker 2021).
Cylindrospermopsin exposure can result in liver and kidney toxicity with impacts on red blood cells and other organs also demonstrated (USEPA 2019). At least four cylindrospermopsin (CYL) structural variants have been identified: 7-epi-CYL, 7-deoxyCYL, 7-deoxyl-desulfo-CYL, and 7-deoxyl-desulfo-12-acetylCYL (Chorus and Welker 2021, USEPA 2019). Toxicity studies have generally been conducted with cylindrospermopsin only, demonstrating inhibition of hepatic protein synthesis via a cytochrome P450-dependent mechanism. For the World Health Organization’s (WHO) provisional guideline values, the sum of all cylindrospermopsins (on a molar basis) is recommended based on limited evidence of similar potency of structural variants to cylindrospermopsin (Chorus and Welker 2021, WHO 2020b). Also see Section 2.4.
An example of a human illness outbreak related to cylindrospermopsin was reported in 1980 in Australia. During this outbreak, people who consumed drinking water from a water body with an HCB experienced vomiting, liver enlargement, and kidney problems. The causative agent was believed to be cylindrospermopsin, based on the identification of Cylindrospermopsis raciborskii in the water body (WHO 2020b).
In 2010, the CDC reported that cylindrospermopsin was present in two of 11 outbreaks in freshwater lakes in 2009 and 2010. People exposed in these outbreaks experienced gastrointestinal symptoms, though it is not known if the symptoms were because of the cylindrospermopsin or because of other cyanotoxins in the water. Anatoxin-a and microcystin were present, and in one case, saxitoxin (Hilborn et al. 2014). Longer term health effects from cylindrospermopsin are currently unknown (Chorus and Welker 2021).
2.3.2 Neurotoxins
Neurotoxins affect the nervous system, often by blocking receptors in a way that can result in respiratory failure. Two examples of neurotoxins are anatoxin-a and saxitoxin. Although anatoxin-a, guanitoxin, and saxitoxin classes all act as neurotoxins, they act by different modes of action (Aráoz, Molgó, and Tandeau de Marsac 2010, Valério, Chaves, and Tenreiro 2010) and therefore have different threshold values, complicating direct comparisons.
Several animal deaths from exposure to cyanobacteria neurotoxins like anatoxin-a have been documented (Chorus and Welker 2021). The neurotoxins can be particularly harmful to dogs, especially because dogs are attracted to the biomass in the water and are likely to mouth or ingest it when it floats to shore. Additional exposure can occur when dogs lick their fur.
2.3.2.1 Anatoxins
Anatoxins have been reported more frequently in benthic blooms than other types of cyanotoxins. According to the World Health Organization, species that are common producers of anatoxin-a are “Anabaena (planktonic species of which are now classified as Dolichospermum), Aphanizomenon (some species of which are now classified as Cuspidothrix and some as Chrysosporum), Raphidiopsis (formerly Cylindrospermopsis), Cylindrospermum, Oscillatoria, Planktothrix, Phormidium, Lyngbya (some species of which are now classified as Microseira and some as Moorea), Tychonema, Blennothrix and Kamptonema” (WHO 2020a). Because extracellular anatoxin-a has a short half-life in the environment, intracellular benthic sources may be important in animal deaths even when it is not found in ambient water samples. Chorus and Welker (2021) reported that cyanobacteria that produce anatoxin-a include species in the orders Nostocales and Oscillatoriales.
Anatoxins have the following characteristics:
- Alkaloid chemical structure (Chorus and Bartram 1999).
- Highly soluble in water.
- Release: Confined to viable cells until cells die and lyse (Chorus and Welker 2021).
- Degradation of extracellular/dissolved anatoxin-a: Dependent on pH. Half-life can be up to 1–2 hours in sunlight in alkaline pH, but in darkness may be about 14 days (Chorus and Bartram 1999, Chorus and Welker 2021).
- Bioaccumulation potential of anatoxin-a is low (National Center for Biotechnology Information 2021c).
- Structural variants: homoanatoxin-a; epoxy and dihydro derivatives.
Anatoxins are alkaloids that are similar to other cyanotoxins. There are several structural variants of anatoxin-a, including homoanatoxin-a, but anatoxin-a is the most studied. Structural variants of anatoxin-a (ATX) include homoanatoxin-a (HTX), dihydroanatoxin-a (dhATX), and dihydrohomoanatoxin-a (dhHTX), which are nicotinic acetylcholine receptor agonists. A recent study comparing dhATX and anatoxin-a toxicity in mice found dhATX was 3–4 times more acutely toxic by oral administration (feeding and gavage) than ATX, although the reverse was true by intraperitoneal injection (Puddick et al. 2021).
Humans have experienced documented illnesses following exposure to anatoxins. Although no deaths have been reported in humans, Hilborn et al. (2014) described three recreational water exposure outbreaks involving human neurological symptoms when anatoxin-a was in the water. Human poisoning following consumption of a marine invertebrate (sea fig) contaminated with anatoxin-a has been documented in France (Biré et al. 2020). Symptoms included difficulty with focusing, double vision, impaired coordination, dizziness, tinnitus, and cramping in the legs and abdomen. These symptoms resolved within 3–24 hours. Similar symptoms were exhibited with earlier sea fig consumption events, but anatoxin-a analysis of leftovers was not conducted (Schmitt et al. 2019).
2.3.2.2 Saxitoxins
Saxitoxins are potent neurotoxins also known as paralytic shellfish poisoning toxins. Saxitoxins in freshwater have been produced by cyanobacteria like Dolichospermum and Raphidioposis (Chorus and Welker 2021, Section 2.4.4.1). Saxitoxins have recently been identified in benthic species of cyanobacteria, such as in Lyngbya (now Microseira) wollei on the St. Lawrence River in Canada (Lajeunesse et al. 2012) and unspecified streams in the United States, and in Scytonema Agardh in New Zealand (Smith et al. 2011).
Saxitoxins have the following characteristics:
- Alkaloid chemical structure.
- Water soluble.
- Stable (Boiling in water does not degrade them.)
- Release: Currently data are lacking on when these cyanotoxins are released from cells (Chorus and Welker 2021).
- Degradation of cyanotoxin: The half-life of saxitoxins is 1–10 weeks, with up to 3 months needed for 90% degradation (Chorus and Welker 2021).
- Bioaccumulation: Saxitoxins bioaccumulate to high levels in sea organisms, particularly filter feeders; however, less is known about freshwater organisms (Chorus and Welker 2021).
- Structural variants: There are 57 identified, with saxitoxin as the parent (WHO 2020d).
Saxitoxins as a class of cyanotoxins refer to 57 structural variants that can block signals in nerve and cardiac cells. Specifically, saxitoxins can bind to and block voltage-gated sodium channels in neuronal cells and, to a lesser extent, the calcium and potassium channels in cardiac cells (USEPA 2019, WHO 2020d). FAO/WHO (2016) identified TEFs for saxitoxin structural variants ranging from 0.01 for C1 and C3 N-sulfocarbamoyl saxitoxins to 2 for neosaxitoxin, relative to saxitoxin as the reference compound (TEF of 1). WHO provisional guideline values for saxitoxin allow for simple addition of concentrations of all saxitoxin structural variants present as a conservative approach unless the more toxic neosaxitoxin is the dominant structural variant present (Chorus and Welker 2021).
Saxitoxins can be found in water or shellfish. In marine water, saxitoxins accumulate in shellfish and are a known poison in this food. In freshwater, saxitoxins are found more often in water, though to date, they appear in lower concentrations than microcystins and anatoxins.
Consumption of seafood contaminated with saxitoxins has caused acute poisonings in humans, with a range of mild to severe symptoms and even death (WHO 2020d). The severity of the response seems to vary by personal sensitivity and dose. Mild symptoms can involve numbness or tingling in the face or lips. A prickly sensation in the extremities, headaches, dizziness, and other gastrointestinal symptoms can also occur. In severe cases, muscle paralysis and respiratory failure can occur (WHO 2020d). Patient recovery from severe saxitoxin poisoning is dependent on the availability of advanced medical care, including mechanical ventilation (see WHO 2020d). Effects of long-term (chronic), low-level exposure have not been well studied (WHO 2020d).
2.3.2.3 Guanitoxin
Guanitoxin, originally identified as anatoxin-a(S), is an organophosphate that irreversibly inhibits acetylcholinesterase in neuromuscular junctions similar to synthetic organophosphate pesticides, but is not known to pass the blood-brain barrier (Chorus and Welker 2021). No structural variants of guanitoxin have been identified. Planktonic cyanobacteria genera Dolichospermum and Sphaerospermopsis (formerly Anabaena and Dolichospermum, respectively) have been identified as guanitoxin producers (Chorus and Welker 2021, Fiore et al. 2020). As of June 2021, no benthic cyanobacteria genera are known producers of guanitoxin.
2.3.2.4 beta-Methylamino-L-alanine (BMAA)
BMAA is a non-proteinogenic amino acid originally discovered in Guam in cycad seeds, but it is also produced by some cyanobacteria and diatoms (Chorus and Welker 2021, Cox, Banack, and Murch 2003, National Center for Biotechnology Information 2021a). BMAA is a neurotoxin that has been postulated as a possible cause of neurodegenerative disorders of aging such as Alzheimer’s disease, amyotrophic lateral sclerosis, and the amyotrophic lateral sclerosis/parkinsonism-dementia complex (ALS-PDC) syndrome of Guam (Chernoff et al. 2017, Chorus and Welker 2021, Ra et al. 2021). This hypothesis is being actively investigated in nonhuman primates, human neural cell lines, and animal models (Silva et al. 2020).
2.3.2.5 Aetokthonotoxin
This neurotoxin was recently identified from an epiphytic cyanobacteria known to grow on Hydrilla, an aquatic weed, in the presence of a bromide source (Breinlinger et al. 2021). It is a biindole alkaloid isolated from the cyanobacterium Aetokthonos hydrillicola. It causes avian vacuolar myelinopathy (AVM) in bald eagles (National Center for Biotechnology Information 2021d).
2.3.3 Dermatoxins and Skin-irritating Compounds
Cyanobacteria dermatoxins can cause dermatitis and skin irritation. Although dermatoxins may be produced by several different species (Chorus and Welker 2021), the genus Lyngbya has traditionally been thought to be the primary producer of many of these cyanotoxins, with lyngbyatoxin A being a recognized cyanotoxin. Dermatoxins are primarily produced in marine cyanobacteria, though dermatoxins from Lyngbya/Microseira genus and others can be found in freshwater.
Aplysiatoxin, debromoaplysiatoxin, and lyngbyatoxins can cause dermal toxicity, and tumor-promoting activity via the protein kinase C activation pathway. When these cyanotoxins are ingested, internal inflammation, necrosis, and hemorrhaging have been documented (Chorus and Welker 2021).
Exposure of skin to elevated amounts of cyanobacteria cells (such as in discolored water, scum, or mats) has been associated with dermal effects such as skin rashes, ear and eye infections, and gastrointestinal distress. The allergic/irritant reactions have been noted in non-cyanotoxin-producing strains and when cyanotoxins are not detected, suggesting that in some circumstances these symptoms are not cyanotoxin-mediated, and may be related to the lipopolysaccharide present on the outside of cyanobacterial cells (USEPA 2019). USEPA did not establish a cell density threshold (cells/mL) as part of its recreational water quality criteria for cyanotoxins given the range in responses and lack of clear dose-response for that measure (USEPA 2019). Chorus and Welker (2021) indicated that while lipopolysaccharides can cause biological activity, there is not clear evidence of a human health threat to the level that cyanotoxins like microcystin may cause.
2.3.4 Other Cyanobacterial Secondary Metabolites
Cyanobacteria produce a wide variety of other substances that appear to produce adverse effects. The CyanoMetDB project provides a comprehensive public database of the 2,010 currently known secondary metabolites from cyanobacteria, many of which lack specific analytical chemistry standards and methods (Jones et al. 2021). Cyanobacterial extracts have been reported to cause toxicity effects that could not be explained by known substances in the extract (Chorus and Welker 2021; see Section 2.10). However, it is not known clearly what causes the effects, which could be related to cyanobacteria metabolites or bacteria that might grow in HCBs. More study might result in identification of more cyanotoxins, and in the interim, it is important to recognize the necessity to avoid HCBs.
2.4 Cyanotoxin Distribution, Stability, and Exposure Considerations
Cyanotoxins are produced within cyanobacterial cells. Most cyanotoxins remain within the cells until the cells break open and die (lysis) due to natural causes or treatment (such as water body management or drinking water processes). The known exception is cylindrospermopsin, which appears to be actively released from intact cells, and up to 90 percent may be extracellular (see Buratti et al. 2017, USEPA 2015a, d, c, 2019, WHO 2020b). For a given cyanotoxin, the portion of intracellular and extracellular cyanotoxin is not known to vary between planktonic and benthic cyanobacteria sources. The relative abundance of both intracellular and extracellular cyanotoxins is an important consideration for monitoring (Section 3) and management/treatment for recreational and drinking water sources (Section 4).
The relative density and distribution of cyanobacterial cells and associated intracellular cyanotoxins do vary more widely, which affects sample cyanotoxin concentrations and potential for exposure. For planktonic cyanobacteria, the abundance and distribution of cells within the water column can change with time of day or season with highest density and concentrations in accumulated scums. Potential exposure would be highest with scums that have the highest density of cells and intracellular cyanotoxins. A water sample containing scum may be analyzed and the subsequent cyanotoxin concentration (in ug/L) compared to a water threshold to address this potential higher exposure.
Benthic cyanobacteria with no to minimal detachment or disturbance may contribute few clumps of cells into the overlying water, resulting in relatively low water concentrations and low potential exposure with incidental water ingestion. For example, a study of Lyngbya wollei toxin (LWTX-1) in Microseira (formerly Lyngbya) wollei mats and overlying water found water concentrations up to 93 ng/L and up to 394 ng/mg dry mass in filament samples (Poirier-Larabie et al. 2020).
With more significant detachment or disturbance of benthic cyanobacteria, the occurrence of floating and stranded clumps would typically increase. In these circumstances, incidental ingestion of floating clumps during recreation or consumption of stranded mat material, such as by dogs and young children, would represent a greater potential exposure. In Appendix B.2, the Zion case study describes how recreational disturbance is simulated during sample collection such that water grab samples incorporate detached, floating material. To accurately evaluate the risk posed by direct ingestion of stranded mat material, cyanotoxin concentrations in mats should be determined in the mat material (μg cyanotoxin/g mat) and compared to an applicable threshold, if available (see Section 2.6).
Researchers are studying how, where, and when cyanotoxins can accumulate in biota such as fish, shellfish and plants, and the subsequent potential for human exposure (Chorus and Welker 2021). Additional research needs are identified in Section 6. Machado et al. (2017) and Lee et al. (2017) reviewed cyanotoxin uptake into plants or onto leaf surfaces through irrigation, relative distribution to different tissues, and potential impacts to plants and their consumers. With fish and shellfish tissues, cyanotoxins generally accumulate to higher levels in the internal organs than the muscle tissue; however, the analytical method used and whether free or covalently bound cyanotoxins (or both) are measured affect the comparability of various studies (Chorus and Welker 2021, Testai, Scardala, et al. 2016). Removal of internal organs and washing produce prior to cooking are generally recommended food practices, but cooking will not break down any cyanotoxins present in produce or meat.
2.4.1 Stability of Intracellular Cyanotoxins
The stability of cyanotoxins within the cells is generally higher than for extracellular cyanotoxins, which are directly subject to abiotic and biotic degradation. Cyanobacteria in dried, preserved herbarium samples (from 1839 to 1950) and samples of cyanobacterial mats from an early 1900s Antarctica expedition still had detectable cyanotoxin-producing genes and cyanotoxins. Martín-Betancor et al. (2017) found freeze-dried Anabaena were viable in a bioluminescent bioassay after 3 years in storage. Dried Microcystis crusts approximately 5–6 months old from a shoreline were toxic in mouse bioassay and had detectable microcystin concentrations with a similar profile to fresh material (Jones, Falconer, and Wilkins 1995). Leaching experiments by Jones, Falconer, and Wilkins (1995) showed that rewetting the crust material led to release of microcystins into surrounding water. This stability in dried materials allows for normalization of mat cyanotoxin concentrations as dry weight through oven or freeze-drying prior to chemical analysis (Section 3.3.4). Instances of dogs intentionally consuming dried mat material and exhibiting subsequent symptoms of cyanotoxin exposure have been documented (Backer et al. 2013).
If cyanobacterial cells are ingested, the cells lyse within the gastrointestinal tract of organisms, resulting in a rapid release of previously intracellular cyanotoxins that can be absorbed into the body. For that reason, cyanotoxins in water or mat are typically analyzed as total concentrations (both intracellular and extracellular) with an initial freeze/thaw or chemical lysis step, and the total concentration is compared to relevant cyanotoxin thresholds. Some devices only sample dissolved cyanotoxins, such as solid phase absorption toxin tracking (SPATT, Section 3.2.4.9).
2.5 Developing Cyanotoxin Toxicity Thresholds for Humans and Animals
2.5.1 Concept for Threshold Equation
Toxicity thresholds are based on the concept that there is a level (threshold or dose) under which no adverse health effects are expected during typical exposure scenarios, and these values are generally expressed as a concentration (amount of cyanotoxin per unit, volume or biomass, ingested). Although the details of deriving numerical threshold values for a cyanotoxin can be complex and presented in various forms, the key elements are toxicity, exposure, and uncertainty factors. The toxicity component generally includes a reference dose concentration derived from toxicological studies. In some threshold calculations, the toxicity component includes specific uncertainty (safety or chemical-specific adjustment) factors, which are dimensionless units, when applying a reference value to another species or broader population or accounting for other unknowns. Chorus and Welker (2021) noted that WHO considers threshold values with high uncertainty factors (greater than 1,000) as provisional and more likely to change based on new information. The exposure component includes estimates for how much of the contaminated water or biomass is ingested, as well as estimates for the size of the person or animal, and in some cases an additional safety factor is included in exposure. For more details on deriving toxicity thresholds for cyanobacteria, see Chorus and Welker (2021, Box 2.1: How are guideline values derived?).
When comparing threshold values from different reports or entities, it is important to consider where differences occur in those calculations. For example, a ten-fold difference in threshold values may be due to an uncertainty or safety factor while both the reference dose (underlying toxicity study) and other exposure values are the same. Cotruvo (2020) compared numerical thresholds for microcystins in drinking water from USEPA (2015a) and WHO (2020c) and detailed several important differences: reference dose study, uncertainty factors, body weight, and ingestion (liters of water consumed per day).
2.5.2 Exposure Duration
Because cyanotoxins vary in mode of action (end point or target) and toxicity can differ for an acute (one-time) exposure or subchronic, multiday exposures, it is important to be aware of the exposure period associated with the threshold and/or guidance values. USEPA (2015a, 2015c, 2015d) human drinking water threshold levels for microcystins and cylindrospermopsin are specified as a 10-day period, whereas WHO human drinking water thresholds for short-term exposure are defined as a period of about 2 weeks (WHO 2020a, b, c, d). Conversely, threshold values for saxitoxin and anatoxin-a are considered acute, and threshold values may be applicable to a single day or single exposure event. Although reference dose values can be applied to a long-term exposure (entire lifetime), it is important to note that this may only be applicable to the primary mode of action (such as liver lesions in microcystins) and may not be protective of other health effects, which warrant more research with long-term studies. The WHO (2020a, 2020b, 2020c, 2020d) guidance calculates a tolerable daily intake (TDI) to represent the amount of a substance (cyanotoxin) that can be ingested daily over a lifetime without substantial health risks. The TDI value can then be applied to other periods or exposures (such as consuming contaminated food).
2.5.3 Human Ingestion Values Applied to Cyanotoxin Thresholds
2.5.3.1 Human Ingestion Values for Water
The calculation of toxicity thresholds requires an estimate of how much water was ingested, either as the primary source of drinking water or through incidental ingestion while recreating.
2.5.3.2 Human Ingestion Values of Mats
To date, we are not aware of any published incidental ingestion rates of benthic mat material for humans during water recreation, or for hand-to-mouth behavior of children who may be handling mat material.
2.5.3.3 Human Ingestion Values for Consumption of Fish and Shellfish
Humans may be exposed to cyanotoxins through food, including consumption of fish and shellfish. Generally, fish consumption for humans is calculated for fillet only, whereas shellfish may be consumed whole.
2.5.4 Ingestion Rates for Domestic Animals
2.5.4.1 Dog Ingestion Values for Water
Dogs may be exposed to cyanotoxins by drinking from contaminated water bodies, retrieving toys or waterfowl in water, licking their coats after swimming in contaminated water, or by any combination of these activities.
2.5.4.2 Livestock Ingestion Values for Water
The amount of water ingested by livestock is a key component of the cyanotoxin water thresholds (ug/L) developed for animal exposure.
2.5.4.3 Dog Ingestion Values of Mats
Like cattle, dogs have been known to eat cyanobacterial crusts or mats on the edge of natural or impounded water bodies (Backer et al. 2013). Consumption of crusts and mats is dangerous given the high concentrations of cyanotoxins found in cyanobacteria cells. Dogs exerting energy during hunting or recreation have higher caloric requirements, which may increase the amount of mat that might be consumed. Dog owners should be alert and avoid areas with crusts or mats because dogs can consume a significant amount in just a few minutes.
2.5.4.4 Livestock Ingestion Values of Mats
Cattle have been known to eat cyanobacterial crusts or mats on the edge of natural or impounded water bodies. This scenario is especially risky considering the high concentrations of cyanotoxins found in cells of cyanobacteria.
2.6 Cyanotoxin Thresholds
2.6.1 Cyanotoxin Thresholds for Humans
2.6.1.1 Drinking Water Thresholds for Humans
The following documentation on cyanotoxin concentrations is for finished drinking water, rather than drinking water source water body concentrations or impacts of drinking water treatment on intra- or extracellular cyanotoxin concentrations. Drinking water treatment process impacts to either cyanobacteria or cyanotoxins are beyond the scope for this paper, but resources are available that discuss these issues at length. For more treatment-specific documentation and recommendations, consult the following:
- World Health Organization Toxic Cyanobacteria in Water, 2nd edition (Chorus and Welker 2021)
- American Water Works Association document covering “Managing Cyanotoxins in Drinking Water: A Technical Guidance Manual for Drinking Water Professionals” (AWWA/WRF 2015)
- USEPA website on managing cyanotoxins in public drinking water systems (USEPA 2021i)
- Association of State Drinking Water Administrators HABs rules and resources page (ASDWA 2021)
The WHO has created drinking water recommendations for several of the most prevalent cyanotoxins (Chorus and Welker 2021) (Table 2-2). Derived values are for the specific cyanotoxin structural variant listed. However, because the majority of structural variants for microcystin, cylindrospermopsin, anatoxin-a, and saxitoxin do not have sufficient published data to establish thresholds, the WHO recommends using the listed threshold values as proxies for total concentration for all structural variants in solution. WHO also lumps the nodularin cyanotoxin class in with microcystins, as the chemical structure is similar and affected tissue and organ end points are the same.
The WHO drinking water values have been widely adopted by several countries, including Brazil, Czech Republic, France, Singapore, Uruguay, and South Africa, among others (Sanseverino et al. 2017).
Table 2‑2. WHO-derived guidance values for drinking water
| Cyanotoxin | Provisional lifetime drinking water guidance value | Provisional short-term drinking water guidance value | Acute drinking water guidance value |
| Microcystin-LR | 1 µg/L | 12 µg/L (3 µg/L children and infants) | * |
| Cylindrospermopsin | 0.7 µg/L | 3 µg/L | * |
| Anatoxin-a | * | 30 µg/L (6 µg/L children and infants) | * |
| Saxitoxin | * | * | 3 µg/L |
In response to detections in surface water, New Zealand has set up guidelines for the most common cyanotoxins in drinking water (Ministry of Health 2019) (Table 2-3). Values for several cyanotoxins and cyanotoxin classes have been derived and are listed below. These values are classified as provisional maximum acceptable values (PMAV) and were derived independent of the WHO by the New Zealand government.
Table 2‑3. New Zealand–derived drinking water thresholds for cyanotoxins
Source: Ministry of Health (2019).
| Cyanotoxin | PMAV* | Remarks |
| Anatoxin-a | 6 µg/L | |
| Anatoxin-a(s)** | 1 µg/L | |
| Homoanatoxin-a | 2 µg/L | |
| Cylindrospermopsin | 1 µg/L | |
| Microcystins | 1 µg/L | Expressed as microcystin-LR toxicity equivalents |
| Nodularin | 1 µg/L | |
| Saxitoxins | 3 µg/L | Expressed as saxitoxin equivalents |
**Now known as guanitoxin.
Canada has developed a microcystin in drinking water guideline value of 1.5 µg/L (Health Canada 2017). Guideline values for other cyanotoxins have not been derived due to the limited amount of data available.
Other countries with cyanotoxin drinking water guideline values include Australia and Finland (Sanseverino et al. 2017). Australia has cyanotoxin values for microcystin-LR (1.3 ug/L), cylindrospermopsin (1 ug/L), and saxitoxin (3 ug/L). Finland has a cyanotoxin value of 1 ug/L for microcystin.
The USEPA has established drinking water health advisory values for microcystin and cylindrospermopsin (USEPA 2015a, b). These values are not considered regulatory but are intended to provide informal technical guidance for state, watershed, and public health authorities when protecting the public from cyanobacterial blooms. These values are not specifically for benthic mat-producing cyanobacteria, but are for concentrations of cyanotoxins in water. Adverse health effects are not anticipated to occur if an individual from each specified age group is exposed to the concentrations listed below for a 10-day exposure period. Either exposure for a longer period of time or to greater concentrations than those specified can put individuals at risk for illness.
Microcystins
- Children under age 6: 0.3 µg/L
- Adults and children 6 years and older: 1.6 µg/L
Cylindrospermopsin
- Children under age 6: 0.7 µg/L
- Adults and children 6 years and older: 3.0 µg/L
Nineteen states have adopted drinking water values for one or more cyanotoxins, as shown in the Figure 2-2. All of those states use either the USEPA’s recommended values for microcystin or they have derived a microcystin value. Seventeen states have a cylindrospermopsin threshold, while five states have anatoxin-a values, and only three states have saxitoxin thresholds. Cited sources for each state’s drinking water cyanotoxin guidance documents are shown in Table 2.4 below.
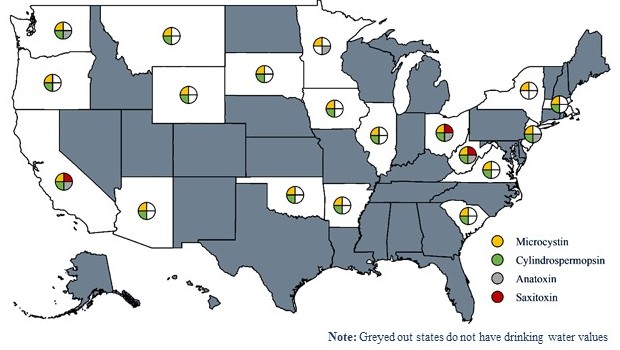
Figure 2‑2. U.S. states that have drinking water threshold values for the four most common cyanotoxins as of June 2021.
Source: Tracy Lund.
Table 2‑4. States that have drinking water limits for the most common cyanotoxins as of June 2021
| State (source) | Microcystin | Anatoxin-a | Saxitoxin | Cylindrospermopsin |
| Arizona (AZ DEQ 2019) | X | X | ||
| Arkansas (AR DEQ 2019) | X | X | ||
| California (CCHAB 2020b) | X* | * | * | X* |
| Illinois (IL EPA 2021) | X | X | ||
| Iowa (IA DNR 2021b) | X | X | ||
| Massachusetts (MassDEP 2021) | X | X | ||
| Minnesota (MN DH 2021) | X | X | ||
| Montana (MT DEQ, MT DPHS, and MT FWP 2018) | X | X | ||
| New Jersey (NJ DEP 2020) | X | X | X | |
| New York (NYS DH 2018) | X | |||
| Ohio (OH EPA 2021) | X | X | X | X |
| Oklahoma (OK DEQ 2021b) | X | X | ||
| Oregon (OR HA 2019a) | X | X | ||
| South Carolina (SC DHEC 2021) | X | X | ||
| South Dakota (SD DOH 2019) | X | X | ||
| Virginia (VA DH 2021) | X | X | ||
| Washington (WA DOH 2020) | X | X | ||
| West Virginia (WV DHHS 2018) | X | X | X | X |
| Wyoming (WY DEQ 2021) | X | X | X | X |
2.6.1.2 Recreational Water Thresholds for Humans
The WHO, Canada, and the United States have developed thresholds for exposure in water based on incidental ingestion during recreation. Thresholds are typically based on total concentration in water and do not usually specify benthic crusts or mats.
The WHO has created recreational recommendations (Table 2-5) for the four most prevalent cyanotoxins (Chorus and Welker 2021). The values derived are for the specific cyanotoxin structural variant listed in ambient water, as opposed to mat material. However, because the majority of structural variants of microcystin, cylindrospermopsin, anatoxin-a, and saxitoxin do not have sufficient published data to establish thresholds, the WHO recommends using the listed threshold values as proxies for total concentration for all structural variants in solution. WHO also lumps the nodularin cyanotoxin class in with microcystins, as the chemical structure is similar and affected tissue and organ end points are similar.
Table 2‑5. WHO recreational guidance values
| Toxin | Provisional recreational water guidance value |
| Microcystin-LR | 24 µg/L |
| Cylindrospermopsin | 6 µg/L |
| Anatoxin-a | 60 µg/L |
| Saxitoxin | 30 µg/L |
Canada has proposed a recreational total microcystins in water guideline value of 10 µg/L. This guideline was still listed as under review as of June 2021 (Health Canada 2020). Guideline values for other cyanotoxins have not been derived due to the limited amount of data available.
The USEPA established recreational health advisory values for microcystin and cylindrospermopsin (Table 2-6) in 2019 (USEPA 2019). These values are not considered regulatory but are intended to provide informal technical guidance for state, watershed, and public health authorities when protecting the public from cyanobacterial blooms in recreational settings. These values are not specifically for benthic mat-producing cyanobacteria but are for water concentration.
Table 2‑6. USEPA-derived cyanotoxin recreational values
| Microcystins | Cylindrospermopsin |
| 8 µg/L | 15 µg/L |
Currently 33 U.S. states have recreational threshold values for one or more cyanotoxins. All of those states use either the USEPA’s recommended values for microcystin or they have derived a microcystin value. Twenty-two states have a cylindrospermopsin threshold, while 17 states have anatoxin-a values, and only ten states have saxitoxin thresholds. Figure 2-3 indicates states that have recreational values for the four most common cyanotoxins.
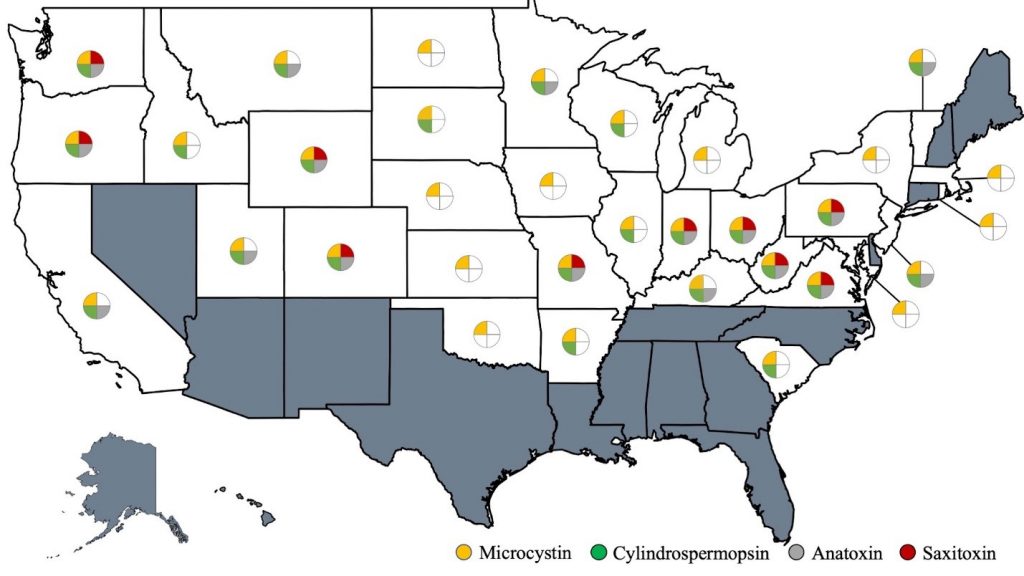
Figure 2‑3. U.S. states that have recreational threshold values for the four most common cyanotoxins as of June 2021.
Source: Adapted from Mehinto et al. (2021).
Table 2-7 highlights which states have identified recreational thresholds for the most common cyanotoxins. It is important to bear in mind that these data are current as of June 2021, and states that have not declared recreational values may do so in the future.
Table 2‑7. States that have recreational thresholds for the most common cyanotoxins as of June 2021
| State (source) | Microcystin | Anatoxin-a | Saxitoxin | Cylindrospermopsin |
| Arkansas (AR DEQ 2019) | X | X | ||
| California (CCHAB 2021b) | X | X | X | |
| Colorado (CO DPHE 2020) | X | X | X | X |
| Idaho (ID DEQ, personal communication) | X | X | ||
| Illinois (IL EPA 2021) | X | X | ||
| Indiana (IN DEM 2021) | X | X | X | X |
| Iowa (IA DNR 2021a) | X | |||
| Kansas (KS DHE 2020) | X | |||
| Kentucky (KY EEC 2019) | X | X | X | |
| Maryland (MD DNR, MD DE, and MD DHMH 2014) | X | |||
| Massachusetts (MassDEP 2021) | X | |||
| Michigan (Kohlhepp 2015) | X | |||
| Minnesota (MN PCA 2021) | X | X | X | |
| Missouri (MO DNR 2021) | X | X | X | X |
| Montana (MT DEQ, MT DPHS, and MT FWP 2018) | X | X | ||
| Nebraska (NE DEE 2021) | X | |||
| New Jersey (NJ DEP 2020) | X | X | X | |
| New York (NYS DEC 2019) | X | |||
| North Dakota (Mehinto et al. 2021) | X | |||
| Ohio (State of Ohio 2020) | X | X | X | X |
| Oklahoma (OK DEQ 2021a) | X | |||
| Oregon (OR HA 2019a) | X | X | X | X |
| Pennsylvania (PA DEP 2021) | X | X | X | X |
| Rhode Island (RI DEM 2019) | X | |||
| South Carolina (SC DHEC 2021) | X | X | ||
| South Dakota (SD DOH 2019) | X | X | ||
| Utah (UT DH and UT DEQ 2020) | X | X | X | |
| Vermont (VT DH 2021) | X | X | X | |
| Virginia (VA DH 2021) | X | X | X | X |
| Washington (WA DOH 2021) | X | X | X | X |
| West Virginia (WV DEP 2021) | X | X | X | X |
| Wisconsin (WI DNR 2021) | X | X | ||
| Wyoming (WY DEQ 2021) | X | X | X | X |
2.6.1.3 Mat Material Thresholds for Human Consumption
Although Cuba and New Zealand have established numerical thresholds for benthic cyanobacteria, those thresholds are based on “percent cover” for the attached portion, and not on mat cyanotoxin thresholds (Chorus 2012, Ibelings et al. 2015). In the United States, three states responded positively to the ITRC Benthic HCB team state survey question regarding cyanotoxin thresholds for benthic mat material, although all evaluate as water concentrations (ug/L), such as with the Zion National Park case study (Appendix B.2), not per mat biomass (ug/g). As noted in Section 6.1, additional evaluation of human exposure to floating or stranded mat material is needed to estimate specific mat biomass thresholds for human health.
2.6.1.4 Integration of Human Recreational Water and Mat Thresholds
As noted above, cyanotoxin concentrations are typically higher in mat material, where cyanobacterial cells with intracellular cyanotoxins are concentrated, than in the overlying water where extracellular cyanotoxins or smaller accumulations of detached benthic cyanobacteria may be present. In addition, some lakes may experience a planktonic bloom and have potentially toxigenic benthic mats also growing in the lake at the same time. Therefore, the integration of water and mat concentrations and the results of comparisons to applicable thresholds should be considered and accounted for in subsequent communication and signage. For example, see the discussion and decision tree developed by the California Cyanobacteria and Harmful Algal Bloom (CCHAB) Network Benthic Signage Subcommittee (CCHAB 2021b) .
The Zion National Park (Appendix B.2) case study demonstrates how cyanotoxin thresholds developed for human exposure to recreation water can applied to a benthic HCB event. Here, they adapted the sampling methodology to mimic recreational exposure risk to a planktonic bloom and capture a reasonable, worst-case scenario. The sampling method was termed “benthic disturbance,” and the protocol calls for artificially disturbing the benthic cyanobacterial colonies in an area to release and suspend benthic cyanobacteria and then collecting the water sample (see further details in Appendix B.2). The benthic disturbance samples are analyzed for anatoxin-a with results reported in concentration per water volume, which can be compared to recreational threshold values. The Zion Benthic HCB Recreational Advisory is tiered based on anatoxin-a recreational thresholds of 15 and 90 µg/L and additional data (i.e., presence of toxigenic cyanobacteria species, SPATT samples). Note that anatoxin-a results from the passive SPATT samples are considered only as detect or nondetect results, as the media in SPATT samplers only sorbs extracellular (dissolved) cyanotoxins.
2.6.1.5 Fish/Shellfish Tissue Thresholds for Human Consumption
Although most guidance values are for cyanotoxins in water or bloom material, some guidance values for human health are available for fish and shellfish tissue concentrations.
- The U.S. Food and Drug Administration (FDA) has a threshold of ≥ 0.8 mg/kg saxitoxin equivalent in fish and shellfish tissue (FDA 2020).
- OEHHA (2012) has thresholds for three cyanotoxins (microcystins, cylindrospermopsin, and anatoxin-a) for recreational fish and shellfish consumption (see Table 2.8).
- In addition, precautions regarding fish and shellfish consumption may be incorporated based on water concentrations as noted below.
Table 2‑8. Cyanotoxin action levels for fish and shellfish
| Microcystins (ng/g tissue, wet weight) | Anatoxin-a (ng/g tissue, wet weight) | Cylindrospermopsin (ng/g tissue, wet weight) | Saxitoxin (ng/g tissue, wet weight) | |
| California, recreational (OEHHA 2012) | 10 | 5000 | 70 | n/a |
| FDA (FDA 2020) | n/a | n/a | n/a | 0.8 |
2.6.2 Cyanotoxin Thresholds for Domestic Animals
2.6.2.1 Water Thresholds for Domesticated Pets and Livestock
Numerical limits established for pets and livestock are not widespread, due to existing regulatory structures, although states commonly include discussions on preventing pets and livestock from entering and drinking from water bodies that appear to be hosting a cyanobacterial bloom.
Some states have developed values for cyanotoxins to inform veterinarians and pet/livestock owners about cyanotoxin risks (Table 2-9). Other states have guideline values for cyanotoxins, such as California, Indiana, Oregon, and Pennsylvania.
It is worth noting that values for pets and livestock are often lower than drinking water threshold values. For instance, Oregon Health Authority’s guidance recommends that pet owners provide alternative water sources for pets when a drinking water advisory is in effect (OR HA 2019b).
Table 2‑9. U.S. state-derived water threshold values for dogs and cattle
| State | Animal and water use | Microcystin | Anatoxin-a | Saxitoxin | Cylindrospermopsin |
| California (OEHHA 2012) | Dog, subchronic water intake | 2 µg/L | 100 µg/L | — | 10 µg/L |
| California (OEHHA 2012) | Cattle, subchronic water intake | 0.9 µg/L | 40 µg/L | — | 5 µg/L |
| Indiana (IN DEM 2021) | Dog recreational | 0.8 µg/L | Any detection | Any detection | 1.0 µg/L |
| Oregon (OR HA 2019a) | Dog recreational | 0.2 µg/L | 0.4 µg/L | 0.02 µg/L | 0.4 µg/L |
| Pennsylvania (PA DEP 2017) | Dog, non-specified | 0.2 µg/L | 0.6 µg/L | 3 µg/L | 0.2 µg/L |
2.6.2.2 Domestic Animal Cyanotoxin Thresholds in Mat Material
In the United States, three states responded positively to the ITRC Benthic HCB team state survey question regarding cyanotoxin thresholds for benthic mat material, but upon further investigation, it appears that only California has cyanotoxin thresholds specifically for dogs and livestock, which are based on cyanotoxin concentration per weight of mat material. Therefore, a summary of California’s cyanotoxin action levels in crusts and mats for dogs and cattle is included below.